Affective information modulates slow-wave- and arousal-like responses during NREM sleep
- PMID: 40676188
- PMCID: PMC12271331
- DOI: 10.1038/s42003-025-08480-3
Affective information modulates slow-wave- and arousal-like responses during NREM sleep
Abstract
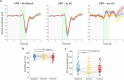
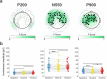
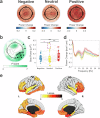
Sleep is characterized by relative disconnection from the external environment and prompt reversibility in response to salient stimuli. During non-rapid eye movement (NREM) sleep, reactive electroencephalographic (EEG) slow waves (K-complexes, KC) are thought to both suppress the processing of external stimuli and open 'sentinel' windows during which further relevant inputs may be tracked. However, the extent to which a stimulus's relevance modulates the KC-related response remains unclear. Here, we investigated the impact of emotional information in human vocal bursts on KC and post-KC activity. Twenty-five young adults were presented with vocal bursts conveying negative, neutral, and positive emotions. We found that affective content influenced the rate, amplitude, and cortical distribution of KCs, as well as post-KC high-frequency activity. These results indicate that KCs are not all-or-none responses and that salient information is not entirely 'quenched' by KCs. These insights offer new perspectives on how sleep continuity and reversibility are regulated.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Anafi, R. C., Kayser, M. S. & Raizen, D. M. Exploring phylogeny to find the function of sleep. Nat. Rev. Neurosci.20, 2 (2019). Article. - PubMed
-
- Sotelo, M. I., Tyan, J., Dzera, J. & Eban-Rothschild, A. Sleep and motivated behaviors, from physiology to pathology. Curr. Opin Physiology15, 159–166 (2020).
-
- Bonnet, M. H. & Arand, D. L. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep. Med. Rev.7, 297–310 (2003). - PubMed
-
- Boon, M. E., van Hooff, M. L. M., Vink, J. M. & Geurts, S. A. E. The effect of fragmented sleep on emotion regulation ability and usage. Cognit. Emot.37, 1132–1143 (2023). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

