Amyloid-β disrupts APP-regulated protein aggregation and dissociation from recycling endosomal membranes
- PMID: 40676215
- PMCID: PMC12361456
- DOI: 10.1038/s44318-025-00497-y
Amyloid-β disrupts APP-regulated protein aggregation and dissociation from recycling endosomal membranes
Abstract
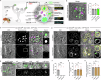
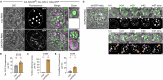
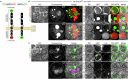
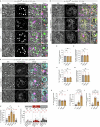
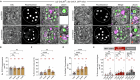
Secretory proteins aggregate into non-soluble dense-core granules in recycling endosome-like compartments prior to regulated release. By contrast, aberrantly processed, secreted amyloid-β (Aβ) peptides derived from amyloid precursor protein (APP) form pathological extracellular amyloidogenic aggregations in late-stage Alzheimer's disease (AD). By examining living Drosophila prostate-like secondary cells, we show that both APP and Aβ peptides affect normal biogenesis of dense-core granules. These cells generate dense-core granules and secreted nanovesicles called Rab11-exosomes via evolutionarily conserved mechanisms within highly enlarged secretory compartments with recycling endosomal identity. The fly APP homologue, APP-like (APPL), associates with these vesicles and the compartmental limiting membrane, from where its extracellular domain modulates protein aggregation. Proteolytic release of this domain permits mini-aggregates to coalesce into a large central dense-core granule. Mutant Aβ expression disrupts this process and compartment motility, and increases aberrant lysosomal targeting, mirroring previously unexplained early-stage pathological events in AD. It also promotes cell-to-cell propagation of these endolysosomal defects, again phenocopying changes observed in AD. Our data therefore demonstrate physiological roles for APP in membrane-dependent protein aggregation, involving molecular mechanisms, which when disrupted by Aβ peptides, trigger Alzheimer's disease-relevant pathologies.
Keywords: Alzheimer’s Disease; Dense-Core Granules; Exosomes; Rab11; Transforming Growth Factor-Beta-Induced.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures







References
-
- August A, Schmidt N, Klingler J, Baumkötter F, Lechner M, Klement J, Eggert S, Vargas C, Wild K, Keller S et al (2019) Copper and zinc ions govern the trans-directed dimerization of APP family members in multiple ways. J Neurochem 151:626–641 - PubMed
MeSH terms
Substances
Grants and funding
- Krebs Scholarship/Biochemical Society (The Biochemical Society)
- 133/075/| John Fell Fund, University of Oxford (John Fell OUP Research Fund)
- 111-2320-B-008-001-MY2/National Science and Technology Council (NSTC)
- 113-2320-B-008-003/National Science and Technology Council (NSTC)
- BB/W015455/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- 097813/11/Z/Wellcome Trust (WT)
- BB/N016300/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/L007096/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- WT_/Wellcome Trust/United Kingdom
- C602/A18974/Cancer Research UK (CRUK)
- BB/R004862/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- BB/W00707X/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- N/A/Balliol Interdisciplinary Institute
- C19591/A19076/Cancer Research UK (CRUK)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

