LRRK2 kinase modulates glucose-stimulated insulin secretion via RAB8 phosphorylation and ciliogenesis
- PMID: 40676250
- PMCID: PMC12270992
- DOI: 10.1007/s00018-025-05810-w
LRRK2 kinase modulates glucose-stimulated insulin secretion via RAB8 phosphorylation and ciliogenesis
Abstract
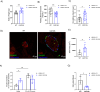
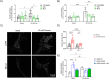
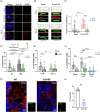
Leucine-rich repeat kinase 2 (LRRK2) encodes a multidomain protein whose mutations have been identified as genetic risk factors for Parkinson's disease (PD), an age-related neurodegenerative disorder. Outside the nervous system, LRRK2 is expressed in multiple tissues, including the endocrine pancreas, but its role here is unknown. Using pharmacological and molecular approaches, we show that LRRK2 kinase activity regulates stimulated insulin secretion by influencing secretory granule trafficking. The PD-associated LRRK2 mutant G2019S, characterized by enhanced kinase activity, increases the basal insulin release in complementary in vitro models and affects the metabolic profile in transgenic mice. Mechanistically, we demonstrate that LRRK2 kinase activity influences the formation of the primary cilium, an antenna-like structure acting as signaling platform to regulate hormones secretion. Specifically, LRRK2 phosphorylates RAB8 in a glucose-dependent manner, facilitating its recruitment to the primary cilium. These findings identify LRRK2 as a regulator of insulin secretion in pancreatic β-cells. Given the role of insulin signaling and glucose homeostasis in the nervous system, our data suggest that LRRK2 may also contribute to PD development through peripheral action.
Keywords: Insulin; LRRK2; β-cell; RABs; Parkinson disease; Primary cilium.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no competing interests. Ethics approval: Animal protocols were approved by the University of Trento and National Ministry of Health (IACUC 793/2016-PR).
Figures