Integrative habitat analysis and multi-instance deep learning for predictive model of PD-1/PD-L1 immunotherapy efficacy in NSCLC patients: a dual-center retrospective study
- PMID: 40676504
- PMCID: PMC12272984
- DOI: 10.1186/s12880-025-01828-5
Integrative habitat analysis and multi-instance deep learning for predictive model of PD-1/PD-L1 immunotherapy efficacy in NSCLC patients: a dual-center retrospective study
Abstract
Background: PD-1/PD-L1 immunotherapy represents the primary treatment for advanced NSCLC patients; however, response rates to this therapy vary among individuals. This dual-center study aimed to integrate habitat radiomics and multi-instance deep learning to predict durable clinical benefits from immunotherapy.
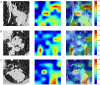
Methods: We retrospectively collected 590 NSCLC patients from two medical centers who received PD-1/PD-L1 inhibitor immunotherapy. Patients from the GMU center were divided into a training cohort (n = 375) and an internal validation cohort (n = 161) for habitat analysis and multi-instance deep learning model development. Patients from the YJ center formed an external testing cohort (n = 54) for model validation. We implemented a DenseNet121-based architecture extracting radiomics features from triplanar (axial/coronal/sagittal) tumor sequences to construct a 2.5D deep-learning dataset. Then, we fuse 2.5D features through multi-instance learning. Additionally, we use K-means clustering to divide the tumor VOI into three subregions to extract radiological features for building a Habitat model. Finally, we use the Extra-Trees classifier to construct MIL, Habitat, and Combined models, the Combined model integrating age factors into the analysis. The primary endpoint was durable clinical benefit. Finally, a separate PD-L1 expression dataset was used to compare the predictive performance of imaging models against PD-L1 status (positive/negative) and expression levels (high/low) to identify the optimal model for predicting immunotherapy clinical benefit.
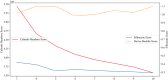
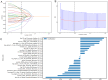
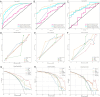
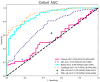
Results: The Combined model combining Habitat, MIL, and patient age demonstrated robust DCB prediction with AUCs of 0.906(95% CI: 0.874-0.936), 0.889(95% CI: 0.826-0.948), and 0.831 (95% CI: 0.710-0.927)in training, validation, and testing cohorts respectively. Comparative analysis revealed all imaging models outperformed PD-L1 expression status (positive/negative) and levels (high/low) in predicting therapeutic response, with Habitat analysis showing superior performance to MIL alone. Notably, peritumoral structural features emerged as significant predictors of treatment efficacy.
Conclusion: This non-invasive predictive framework provides clinically actionable insights for immunotherapy stratification, potentially overcoming limitations of current biomarker testing while highlighting the prognostic value of spatial tumor heterogeneity analysis.
Keywords: Deep learning; Habitat analysis; Immunotherapy; Multiple instance learning; Non-small cell lung cancer; PD-L1 expression.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (identifier: KY-2022-301). The ethics committee waived the requirement for informed consent due to the retrospective nature of the study using anonymized data. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
2.5D deep learning radiomics and clinical data for predicting occult lymph node metastasis in lung adenocarcinoma.BMC Med Imaging. 2025 Jul 1;25(1):225. doi: 10.1186/s12880-025-01759-1. BMC Med Imaging. 2025. PMID: 40597741 Free PMC article.
-
PD-1/PD-L1 inhibitors monotherapy vs. combination therapy in elderly advanced NSCLC: a real-world study and nomogram for survival prognosis.BMC Pulm Med. 2025 Jul 23;25(1):350. doi: 10.1186/s12890-025-03791-x. BMC Pulm Med. 2025. PMID: 40702519 Free PMC article.
-
Prediction of PD-L1 expression in NSCLC patients using PET/CT radiomics and prognostic modelling for immunotherapy in PD-L1-positive NSCLC patients.Clin Radiol. 2025 Jul;86:106915. doi: 10.1016/j.crad.2025.106915. Epub 2025 Apr 2. Clin Radiol. 2025. PMID: 40375402
-
PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review.Lung Cancer. 2017 Oct;112:200-215. doi: 10.1016/j.lungcan.2017.08.005. Epub 2017 Aug 10. Lung Cancer. 2017. PMID: 29191596
References
-
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–39. - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr., Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. - PubMed
-
- Lee SM, Schulz C, Prabhash K, Kowalski D, Szczesna A, Han BH, Rittmeyer A, Talbot T, Vicente D, Califano R, et al. First-line Atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. 2023;402(10400):451–63. - PubMed
-
- West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37. - PubMed
-
- Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, et al. Atezolizumab for First-Line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

