Efficacy and safety of fecal microbiota transplantation in the management of parkinson's disease: a systematic review
- PMID: 40676526
- PMCID: PMC12269262
- DOI: 10.1186/s12883-025-04105-8
Efficacy and safety of fecal microbiota transplantation in the management of parkinson's disease: a systematic review
Abstract
Background: Parkinson's disease (PD) involves progressive neurodegeneration with motor and non-motor symptoms. Gut microbiota alterations are implicated in PD pathogenesis, leading to interest in fecal microbiota transplantation (FMT) as a therapeutic option. This systematic review assesses the efficacy and safety of FMT in managing PD symptoms.
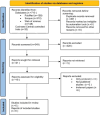
Methods: We conducted a comprehensive search across PubMed, Scopus, Web of Science, and Cochrane Central Controlled trials databases. Studies were screened based on predetermined inclusion criteria, focusing on randomized controlled trials (RCTs) involving FMT in PD patients. Two reviewers independently performed the data extraction and quality assessment. Key outcomes included improvements in motor and non-motor symptoms, quality of life, and adverse effects.
Results: Five RCTs involving 157 patients met the inclusion criteria. Some studies reported improvements in motor and non-motor symptoms, particularly with colonic FMT, while others found no significant benefit. One trial observed motor function worsening. FMT was generally well-tolerated, with mild and transient gastrointestinal side effects.
Conclusion: FMT may relieve PD symptoms, but findings are inconsistent. Larger trials with standardized protocols are needed to determine its long-term efficacy and safety.
Keywords: Clinical trials; Fecal microbiota transplantation; Gut microbiota; Motor symptoms; Non-motor symptoms; Parkinson’s disease; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study. Consent for publication: All authors’ consent has been obtained by the corresponding author for publication. Competing interest: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

