Multi-ancestry genome-wide meta-analysis of 56,241 individuals identifies known and novel cross-population and ancestry-specific associations as novel risk loci for Alzheimer's disease
- PMID: 40676597
- PMCID: PMC12273372
- DOI: 10.1186/s13059-025-03564-z
Multi-ancestry genome-wide meta-analysis of 56,241 individuals identifies known and novel cross-population and ancestry-specific associations as novel risk loci for Alzheimer's disease
Abstract
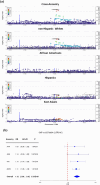
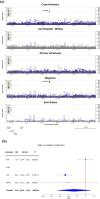
Background: Limited ancestral diversity has impaired our ability to detect risk variants more prevalent in ancestry groups of predominantly non-European ancestral background in genome-wide association studies (GWAS). We construct and analyze a multi-ancestry GWAS dataset in the Alzheimer's Disease Genetics Consortium (ADGC) to test for novel shared and population-specific late-onset Alzheimer's disease (LOAD) susceptibility loci and evaluate underlying genetic architecture in 37,382 non-Hispanic White (NHW), 6728 African American, 8899 Hispanic (HIS), and 3232 East Asian individuals, performing within ancestry fixed-effects meta-analysis followed by a cross-ancestry random-effects meta-analysis.
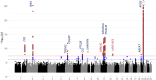
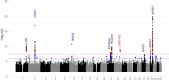
Results: We identify 13 loci with cross-population associations including known loci at/near CR1, BIN1, TREM2, CD2AP, PTK2B, CLU, SHARPIN, MS4A6A, PICALM, ABCA7, APOE, and two novel loci not previously reported at 11p12 (LRRC4C) and 12q24.13 (LHX5-AS1). We additionally identify three population-specific loci with genome-wide significance at/near PTPRK and GRB14 in HIS and KIAA0825 in NHW. Pathway analysis implicates multiple amyloid regulation pathways and the classical complement pathway. Genes at/near our novel loci have known roles in neuronal development (LRRC4C, LHX5-AS1, and PTPRK) and insulin receptor activity regulation (GRB14).
Conclusions: Using cross-population GWAS meta-analyses, we identify novel LOAD susceptibility loci in/near LRRC4C and LHX5-AS1, both with known roles in neuronal development, as well as several novel population-unique loci. Reflecting the power of diverse ancestry in GWAS, we detect the SHARPIN locus with only 13.7% of the sample size of the NHW GWAS study (n = 409,589) in which this locus was first observed. Continued expansion into larger multi-ancestry studies will provide even more power for further elucidating the genomics of late-onset Alzheimer's disease.
Keywords: Alzheimer disease; GWAS meta-analysis,; Genome-wide association study (GWAS); Multi-ancestry; Population-specific.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: For our IRB to compile the de-identified data and conduct analyses, the ADGC protocol is reviewed and approved by the University of Pennsylvania IRB #8 (Federalwide Assurance #00004028). Consent for publication: Not applicable. Competing interests: J.A.P. has received compensation for serving as a section editor for Springer Nature and a grant reviewer with the Department of Defense and Research Grants Council of Hong Kong. M.S.A. is an advisor to Eli Lilly. L.G.A. receives compensation as a consultant for Biogen, Two Labs, IQVIA, NIH, Florida Department of Health, NIH Biobank, Eli Lilly, GE Healthcare, and Eisai; has received compensation for lectures, etc. from AAN, MillerMed, AiSM, and Health and Hospitality; and has received travel and meeting support from the Alzheimer’s Association; she also participates on Data Safety Monitoring or Advisory boards for IQVIA, NIA R01 AG061111, the UAB Nathan Shock Center, and the New Mexico Exploratory ADRC; she has received compensation for leadership roles in the Medical Science Council Alzheimer Association Greater IN Chapter, the Alzheimer Association Science Program Committee, and the FDA PCNS Advisory Committee; she also has stock or stock options Cassava Neurosciences and Golden Seeds; and has received materials support from AVID Pharmaceuticals, Life Molecular Imaging, and Roche Diagnostics. S.E.A. has received honoraria and/or travel expenses for lectures from Abbvie, Eisai, and Biogen and has served on scientific advisory boards of Corte, has received consulting fees from Athira, Cassava, Cognito Therapeutics, EIP Pharma and Orthogonal Neuroscience, and has received research grant support from NIH, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Abbvie, Amylyx, EIP Pharma, Merck, Janssen/Johnson & Johnson, Novartis, and vTv. S.Asthana reported receiving grants from National Institute on Aging/National Institutes of Health, Genentech, Merck, Toyoma Chemical, and Lundbeck outside the submitted work. L.L.B. has served as deputy editor for Alzheimer’s and Dementia for the Alzheimer’s Association. D.A.B. is a consultant for Biogen, Inc. B.F.B. has received institutional support from LBDA; is a member of the Scientific Advisory Boards of the Tau Consortium (funded by the Rainwater Charitable Foundation), AFTD, LBDA, and GE Healthcare; is a member of the Data Safety Monitoring Board of trial involving mesenchymal stem cells in MSA. J.D.B has received honoraria from serving on the Scientific Advisory Board and Speaker’s Bureau of Biogen, Celgene, EMD Serono, Genentech and Novartis; has received research support from AbbVie, Alexion, Alkermes, Biogen, Celgene, Sanofi Genzyme, Genentech, Novartis and TG Therapeutics. A.L.B. has received financial support from NIH, the Association for Frontotemporal Degeneration, the Bluefield Project, the Rainwater Charitable Foundation, Regeneron, Eisai and Biogen; and has served as a paid consultant for AGTC, Alector, Amylyx, AviadoBio, Arkuda, Arrowhead, Arvinas, Eli Lilly, Genentech, LifeEdit, Merck, Modalis, Oligomerix, Oscotec, Transposon and Wave. J.M.B. is compensated as a consultant for Stage 2 Innovations; and has received honoraria and travel support for speaking from Astra-Zeneca. J.D.Buxbaum is a consultant to BridgeBio and to Rumi; holds a patent for IGF- 1 in Phelan-McDermid syndrome; holds an honorary professorship from Aarhus University Denmark; receives research support from Takeda and Oryzon; and is a journal editor for Springer Nature. C.Cao has a patent pending for melatonin-insulin-THC (MIT) treatment; and serves as a scientific consultant for MegaNano Biotech, Inc. C.M.C. has received grants from the National Institutes of Health, Eisai, Eli Lilly, Veterans Affairs; has received nonfinancial support from Amarin; has received data safety monitoring board/travel/advisory board honoraria from Alzheimer's Association, National Institutes of Health, and American Fed Aging Res Beeson Program. J.C. is currently employed as a senior scientist at Takeda Pharmaceuticals, Inc.; the company did not influence the study design, analyses, or interpretation of the results presented in this manuscript. C.Cruchaga has received research support from GSK and Eisai, is a member of the advisory boards of Vivid Genomics and Circular Genomics, and owns stocks. D.W.D. is an editorial board member for Acta Neuropathologica, Brain, Brain Pathology, Neuropathology and Applied Neurobiology, Annals of Neurology, Neuropathology, and is an Editor for the International Journal of Clinical and Experimental Pathology and for the American Journal of Neurodegenerative Disease; and receives support Mangurian Foundation and the Rainwater Charitable Foundation. R.S.D. is an employee of F. Hoffman-La Roche, Ltd. and Genentech, Inc.; and owns or has stock options in F. Hoffmann-La Roche, Ltd. N.E.-T. receives research support from the NIH; is a member of multiple Scientific Advisory Boards including the Framingham Heart Study Executive Committee, Cytox, and the NIH TREAT-AD Consortium External Advisory Board Member; has patents pending for Human Monoclonal Antibodies Against Amyloid Beta Protein and Their Use as Therapeutic Agents Application, and RNAi against targets in Progressive Supranuclear palsy; is an Editorial Board Member for the American Journal of Neurodegenerative Disease and Alzheimer's & Dementia; and receives research support from Florida Health Ed and Ethel Moore Alzheimer's Disease Research Program and an Alzheimer's Association Zenith Award. T.M.F. has received honoraria and travel support from the External Advisory Boards for Alzheimer Disease Research Centers that might also be a site for the LEADS study. D.R.G. serves on Data Safety Monitoring Boards for Cognition Therapeutics and Proclara Biosciences; and is an Editor of Alzheimer’s Research & Therapy. B.G. has consulted for Piramal Imaging. A.M.G is a member of the Scientific Advisory Boards/Scientific Research Boards for Genentech, Muna Therapeutics, and Denali Therapeutics. N.R.G.-.R. has received royalties for an article in UpToDate; and has received research support for multi-center studies at Eli Lilly & Company, Biogen, and AbbVie. R.C.G. has received compensation as an advisor for AIA, Grail, Humanity, Kneed Media, Plumcare, UnitedHealth, Verily, VibrentHealth, Wamberg; and is co-founder of Genome Medical, Inc. J.Hardy is supported by the UK Dementia Research Institute, which receives its funding from DRI, Ltd., funded by the UK Medical Research Council, Alzheimer's Society, and Alzheimer's Research UK; and is also supported by the MRC, Wellcome Trust, the Dolby Family Fund, and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. T.H. is a member of a scientific advisory board for Vivid Genomics. L.S.H. is the Web Editor for JAMA Neurology. B.T.H. has a family member who works at Novartis, and owns stock in Novartis; and serves on the Scientific Advisory Board of Dewpoint and owns stock; and serves on a Scientific Advisory Board or is a consultant for AbbVie, Aprinoia Therapeutics, Arvinas, Avrobio, Axial, Biogen, BMS, Cure Alz Fund, Cell Signaling, Eisai, Genentech, Ionis, Latus, Novartis, Sangamo, Sanofi, Seer, Takeda, the US Dept of Justice, Vigil, Voyager; and receives research support for his laboratory from research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, and the JPB Foundation, and through sponsored research agreements from Abbvie, BMS, and Biogen. G.P.J. was the 2022 Past President of the American Society of Human Genetics. J.H.K. has been a consultant for Biogen. E.B.L. receives royalties from contributions to UpToDate. J.B.L. is a member of the Scientific Advisory Board of Vaxxinity and has received grant support from Biogen and GE Healthcare. A.I.L. is a founder of EmTheraPro. R.B.L. has received research support from the National Institutes of Health, the FDA, and the National Headache Foundation; serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Dr. Reddy’s Laboratories (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Lundbeck, Merck, Novartis, Teva, Vector, and Vedanta Research; receives royalties from Wolff’s Headache, 8 th edition (Oxford University Press, 2009), and Informa; and holds stock in Biohaven and Manistee. D.C.M. receives NIH funding; is the inventor of the FCI-SF and the UAB Research Foundation (UABRF); owns the FCI-SF through copyright and trademark (FCAP); has previously received royalty and consulting income from UABRF licensed use and sale of the FCI-SF; and is currently a consultant on an unaffiliated NIH grant using the FCI-SF. A.V.M. is a council member of the Alzheimer's Association International Research Grants Program, on the steering committee of the Alzheimer's Disease Cooperative Study, and on the editorial boards of Alzheimer's & Dementia: Translational Research and Clinical Interventions and the Journal of Neuro-ophthalmology. S.I.M. is an employee of Pfizer, Inc.; the company did not influence the study design, analyses, or interpretation of the results presented in this manuscript. B.L.M. has received grant support from NIH, the Bluefield Project, and the Rainwater Charitable Foundation; has received royalties from books published by Cambridge University Press, Elsevier, Inc., Guilford Publications, Inc., Johns Hopkins Press, Oxford University Press and Taylor & Francis Group; has received honorarium for serving as a member of the Scientific Advisory Board of the Alzheimer’s Disease Research Center (ADRC) at Massachusetts General Hospital, Stanford University, and the University of Washington; and has received consulting fees from Genworth. J.W.M. receives compensation as Associate Editor for the journal Nutrition Reviews; and has received within the last 3 years consulting compensation from Church and Dwight, Inc.; and is a producer and seller of consumer goods including vitamin supplements. J.C.M is a consultant for clinical trials of antidementia drugs from Eli Lilly and Company, Biogen, and Janssen; is a consultant for the Barcelona Brain Research Center (BBRC) and the TS Srinivasan Advisory Board; is an advisory board member for the Cure Alzheimer’s Fund Research Strategy Council. S.E.O. has multiple pending and issued patents on blood biomarkers for detecting and precision medicine therapeutics in neurodegenerative diseases; and is a founding scientist of Cx Precision Medicine, Inc. and owns stock options. R.C.P. is chair for the data monitoring committee for Pfizer and Janssen Alzheimer Immunotherapy and is a consultant for GE Healthcare and Roche. W.W.P. is a co-inventor of and holds a patent for WO/2018/160496, related to the differentiation of human pluripotent stem cells into microglia. E.M.R. is a scientific advisor to Alzheon, Aural Analytics, Denali, Retromer Therapeutics, and Vaxxinity and a co-founder and advisor to ALZPath. J.M.R. receives research support from Avid Pharmaceuticals. R.N.R. is the Editor of JAMA Neurology. M.S. has received grants from the Icahn School of Medicine at Mount Sinai and the US Department of Veterans Affairs Veterans Health Administration. A.J.S. has received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in-kind contribution of PET tracer precursor), is a member of scientific advisor boards for Bayer Oncology and Eisai and of the dementria advisory board and Siemens Medical Solutions USA, is a member of the National Heart, Lung, and Blood Institute MESA observational study monitoring board, and is part of the editorial office support as editor-in-chief for Brain Imaging and Behavior for Springer-Nature Publishing. J.A.S. has received consulting fees from AVID, Alnylam Pharmaceuticals, and Cerveau Technologies. L.S.S. has received personal fees from AC Immune, Athira, BioVie, Eli Lilly, Lundbeck, Merck, Neurim Ltd., Novo-Nordisk, Otsuka, Roche/Genentech within the past year; and research grants from Biogen, Eisai, and Eli Lilly and Company. W.W.S. serves as a paid consultant to Biogen Idec and has received grant support from NIH, the Association for Frontotemporal Degeneration, the Bluefield Project, the Rainwater Charitable Foundation, and the Chan-Zuckerberg Initiative. S.A.S. has received an unrestricted research grant from Mars, Inc. R.A.S. has received grants from the National Institutes of Health and the from Concussion Legacy Foundation; and received compensation from Biogen and Lundbeck; and has received royalties received from Psychological Assessment Resources for published neuropsychological tests; and has stock options as a member of the board of King Devick Technologies. D.L.S. has received research support from NIH and Eisai, has participated as a paid member of a DSMB or adjudication committee with Acadia, Avanir, Janssen, and Otsuka, and has received consulting fees from Avanir and NovoNordisk. R.E.T. has received patents for gamma-secretase modulators for exploring treatment of Alzheimer's disease. H.W. has received support from the TEVA speaker's bureau. T.S.W. is as a cofounder of revXon. C.B.W. has received royalties from UpTo Date for 2 chapters; has done legal consulting for the law firms of Abali, Milne, and Faegre Baker Daniels; is a consultant for Merck and Co; and does stroke adjudication for a National Institutes of Health clinical trial. L.A.F. has received institutional support from Mass Mutual Insurance. G.T., W.-P.L., Y.Y.L., P.P.K., J.B.M., J.A.P., R.M.S., X.Z., M.S.A., R.L.A., L.G.A., S.E.A., S.Asthana, C.T.B., R.C.B., L.L.B., S.B., T.G.B., J.T.B., D.Beekly, B.B., D.Bennett, T.D.B., D.A.B., B.F.B., J.D.B., A.L.B., J.M.B., J.D.Buxbaum, C.Cao, C.S.C., C.M.C., M.M.C., H.C.C., S.Craft, P.K.C., E.A.C., C.Cruchaga, M.L.C., P.L.D., C.D., J.C.D., M.Dick, D.W.D., R.S.D., R.D., N.E.-T., D.A.E., D.W.F., V.F., T.M.F., M.P.F., D.R.G., A.G., M.G., D.H.G., B.G., A.M.G., N.R.G.-R., R.C.G., H.H., O.Harari, J.Hardy, T.H., L.S.H., R.M.H., M.J.H., B.T.H., G.P.J., M.I.K., A.K., J.S.K., J.A.K., C.D.K., A.Khaleeq, N.W.K., J.H.K., W.K., E.B.L., J.B.L., A.I.L., A.P.L., R.B.L., M.W.L., O.L.L., K.L.L., C.G.L., M.E.F., D.C.M., E.R.M., D.C.Mash, E.M., A.V.M., W.C.M., A.C.M., M.Mesulam, B.L.M., C.A.M., J.W.M., T.J.M., J.C.M., S.Mukherjee, A.J.M., S.E.O., H.L.P., V.P., E.P., R.C.P., W.W.P., E.M.R., J.M.R., E.D.R., M.Rodriguear, R.N.R., M.S., A.J.S., J.A.S., L.S.S., W.W.S., S.A.S., R.A.S., S.M.S., D.S., R.E.T., D.W.T., V.M.VD., L.J.VE., J.M.V., B.N.V., E.M.W., T.S.W., C.B.W., S.G.Y., B.W.K., W.B., L.-S.W., L.A.F., J.L.H., R.M., M.A.P.-V., G.D.S., G.R.J., C.R., and A.C.N. have received grant funding from the National Institutes of Health, including from the National Institute on Aging and others. E.R. has received grant funding from the Canadian Institutes for Health Research.
Figures

References
-
- Alzheimer's Association. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598–695. - PubMed
-
- Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P50 AG008671/AG/NIA NIH HHS/United States
- UL1 RR029893/RR/NCRR NIH HHS/United States
- R56 AG074527/AG/NIA NIH HHS/United States
- R56 AG064877/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P50 MH060451/MH/NIMH NIH HHS/United States
- R01 AG054060/AG/NIA NIH HHS/United States
- R01 AG064877/AG/NIA NIH HHS/United States
- R01 AG022374/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- U19 AG074865/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- U01 HG006375/HG/NHGRI NIH HHS/United States
- U01 AG058654/AG/NIA NIH HHS/United States
- U54 AG052427/AG/NIA NIH HHS/United States
- RC2 AG036528/AG/NIA NIH HHS/United States
- P30 AG028377/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 AG035137/AG/NIA NIH HHS/United States
- R01 AG009029/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P50 AG005128/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 AG031581/AG/NIA NIH HHS/United States
- R01 AG009956/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- R01 AG067501/AG/NIA NIH HHS/United States
- RC2 AG036650/AG/NIA NIH HHS/United States
- R01 AG019085/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- U01 HG008657/HG/NHGRI NIH HHS/United States
- R01 AG013616/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- R01 AG030146/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 NS039764/NS/NINDS NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- U24 AG056270/AG/NIA NIH HHS/United States
- RC2 AG036502/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- U01 AG057659/AG/NIA NIH HHS/United States
- R01 MH080295/MH/NIMH NIH HHS/United States
- R01 AG028786/AG/NIA NIH HHS/United States
- KL2 RR024151/RR/NCRR NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- U01 AG062943/AG/NIA NIH HHS/United States
- R01 AG012101/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- R01 AG042437/AG/NIA NIH HHS/United States
- U24 AG041689/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- R01 AG033193/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R01 AG032990/AG/NIA NIH HHS/United States
- P01 AG060882/AG/NIA NIH HHS/United States
- U01 AG052410/AG/NIA NIH HHS/United States
- R01 CA129769/CA/NCI NIH HHS/United States
- U01 AG010483/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- P01 AG002219/AG/NIA NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- P50 AG005144/AG/NIA NIH HHS/United States
- R01 AG041232/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- R01 AG048927/AG/NIA NIH HHS/United States
- R01 AG020688/AG/NIA NIH HHS/United States
- R01 AG022018/AG/NIA NIH HHS/United States
- R01 AG030653/AG/NIA NIH HHS/United States
- P20 MD000546/MD/NIMHD NIH HHS/United States
- R01 AG017173/AG/NIA NIH HHS/United States
- R01 AG025259/AG/NIA NIH HHS/United States
- RF1 AG057519/AG/NIA NIH HHS/United States
- U01 HG004610/HG/NHGRI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P30 AG066468/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P50 AG016582/AG/NIA NIH HHS/United States
- R01 AG041718/AG/NIA NIH HHS/United States
- U01 AG066767/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- RF1 AG061351/AG/NIA NIH HHS/United States
- R01 AG072474/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- U19 AG066567/AG/NIA NIH HHS/United States
- R01 AG026916/AG/NIA NIH HHS/United States
- P50 AG033514/AG/NIA NIH HHS/United States
- R01 NS059873/NS/NINDS NIH HHS/United States
- R01 AG070864/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

