Diagnostic performance of Pneumonia multiplex PCR in critically ill immunocompromised patients
- PMID: 40676679
- PMCID: PMC12272964
- DOI: 10.1186/s13054-025-05528-y
Diagnostic performance of Pneumonia multiplex PCR in critically ill immunocompromised patients
Abstract
Background: Admissions of immunocompromised patients to intensive care units (ICUs) are on the increase. The main reason for admission is acute respiratory failure, predominantly of infectious origin. In such circumstances, early and appropriate antibiotic therapy guarantees a better prognosis. Rapid diagnostic techniques such as multiplex polymerase chain reaction (PCR) have shown their value in both diagnosis and treatment in immunocompetent patients. To date, little data are available on immunocompromised patients.
Methods: In this retrospective, single-center study, we analyzed data from critically ill immunocompromised patients admitted for acute respiratory failure requiring invasive ventilation, in whom a respiratory specimen was taken and processed simultaneously by BioFire FilmArray Pneumonia Panel multiplex PCR (BFPPm PCR) and conventional culture (CC). Samples had to be taken from deep respiratory tracts less than 48 h after mechanical ventilation. The primary endpoint was the evaluation of the diagnostic performance of BFPP mPCR compared with CC. The secondary endpoint was the therapeutic impact of the results of BFPP mPCR.
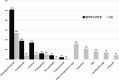
Results: One hundred and fourteen patients were included, with immunosuppression mainly of a hematological (35.1%) and oncological (35.1%) nature. The mPCR positivity rate was 36.8%, with the majority identifying enterobacteria (51%) and a median turnaround time of between 2h30 and 4 h. Comparison of rapid techniques with CC showed sensitivity of 89%, specificity of 83%, predictive positive value of 52% and negative predictive value of 98%. Concordance between the two techniques was complete in 84.2% of cases. mPCR enabled antibiotic therapy to be modified in 17.5% of cases, mainly de-escalation.
Conclusion: The use of mPCR in the diagnosis of pneumonia in immunocompromised patients shortens the time required to obtain results, and is particularly effective in eliminating the presence of multi-resistant germs. Bacteria detected in culture and not included in the mPCR spectrum were mostly bacteria of low pathogenicity or sensitive to the antibiotics usually prescribed. The mPCR technique could reduce exposure to broad-spectrum antibiotics in this population.
Keywords: Acute respiratory failure; Antibiotic; Immunocompromised; Intensive care unit; Multiplex polymerase chain reaction; Pneumonia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The original study protocol was approved by the Institute Review Board of the Montpellier University Hospital (IRB-MTP_2023_09_20230143; dated 09/20/2023) and complied with French health authorities. An information note was given to each patient or their next of kin, and written informed consent of non-opposition was obtained from all patients or their proxies. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Comment in
-
Distinct pathogen spectrum in immunocompromised patients with severe pneumonia.Crit Care. 2025 Oct 8;29(1):425. doi: 10.1186/s13054-025-05704-0. Crit Care. 2025. PMID: 41063269 Free PMC article. No abstract available.
References
-
- Legoff J, Zucman N, Lemiale V, Mokart D, Pène F, Lambert J, et al. Clinical significance of upper airway virus detection in critically ill hematology patients. Am J Respir Crit Care Med. 2019;199:518–28. - PubMed
-
- Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium–a groupe de recherche respiratoire En réanimation onco-hématologique study. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:2810–8. - PubMed
-
- Darmon M, Bourmaud A, Georges Q, Soares M, Jeon K, Oeyen S, et al. Changes in critically ill cancer patients’ short-term outcome over the last decades: results of systematic review with meta-analysis on individual data. Intensive Care Med. 2019;45:977–87. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

