Sex-specific changes in energy demand during the preplaque stage in a transgenic Alzheimer's mouse model
- PMID: 40676711
- PMCID: PMC12273039
- DOI: 10.1186/s13293-025-00737-0
Sex-specific changes in energy demand during the preplaque stage in a transgenic Alzheimer's mouse model
Abstract
Background: Cognitive deficits and brain glucose hypometabolism, lipid peroxidation and mitochondrial dysfunction are early pathological events in murine models and patients with Alzheimer's disease (AD). Data from our previous research indicate that transgenic mice of the APP23 line, a murine AD model, exhibited higher energy expenditure and mitochondrial dysregulation in the liver as early as 3 months of age, which is considered the preplaque stage. Since women have a higher risk and mortality rate for AD, with potential sex-specific confounders as longevity, biological, genetic, and social factors also needing to be considered, sex differences in energy metabolism in AD remain insufficiently investigated.
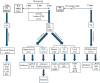
Methods: Here, we investigated sex-specific differences in mitochondrial respiration and metabolic profiles of 3-4-month-old, preplaque APP23 transgenic mice, in which we did not detect inflammatory signals and pathological amyloid-beta (Aß) plaques in brain or liver. Their mitochondrial respiration was assessed measuring oxygen consumption rates in isolated primary hepatocytes, stromal vascular cells (SVCs) and re-differentiated adipocytes. Furthermore, we analyzed energy balance, including food intake, locomotor activity, energy expenditure and fecal calorie loss.
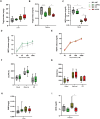
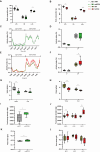
Results: We observed an upregulation of hepatic mitochondrial respiration in preplaque APP23 females. Female-derived SVCs and differentiated adipocytes improved mitochondrial flexibility with palmitate loading in vitro, which was in line with decreased plasma triglycerides in preplaque APP23 females in vivo. However, no differences in mitochondrial respiration were detected in hepatocytes and re-differentiated adipocytes derived from male APP23 mice. Furthermore, we corroborated an increased mortality during the preplaque stage, particularly in females, which exhibited reduced hyperactivity and caloric intake before death compared to survivors.
Conclusions: Our data demonstrate that preplaque APP23 female mice have disequilibrated mitochondrial oxidation in hepatocytes and adipocytes as well as higher energy expenditure due to increased activity before AD manifestation. In contrast, male APP23 mice did not exhibit such metabolic changes. Constant excessive energy loss and limited calorie supply potentially contribute to the higher risk of mortality, especially in APP23 females during young adulthood. Alzheimer's disease (AD) affects men and women differently, with women at higher risk and mortality. This study explored sex differences in energy metabolism using APP23 transgenic mice, a model of AD, at young age (3-4 months) - before pathological amyloid-beta (Aß) plaques develop in the brain and liver. Female APP23 mice showed increased mitochondrial activity in liver and fat cells, higher energy expenditure, and more movement while eating less. They also excreted more energy in their feces. Notably, female APP23 mice had a lower survival rate than males. Before death, they became less active and ate even less, suggesting an inability to maintain energy balance. These findings indicate that female APP23 mice experience excessive energy loss, which may contribute to early mortality. Understanding these sex-specific metabolic differences could provide new insights into AD progression and highlight the need for targeted treatments.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Animal experiments were performed in accordance with Guidance on the operation of the Animals (Scientific Procedures) Act 1986 and associated guidelines, EU Directive 2010/63 and complied with institutional ethical and ARRIVE guidelines and approved by Landesamt für Gesundheit und Soziales Berlin (T0180/16) and Forschungseinrichtung für Experimentelle Medizin Charité (T-CH19/21). Additionally, previously stored samples from an approved experiment (G0074/16) were analyzed. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

