GSK-3β and mTOR Phosphorylation Mediate the Reversible Regulation of Hypomagnetic Field on Adult Neural Stem Cell Proliferation
- PMID: 40676806
- PMCID: PMC12271677
- DOI: 10.1111/ejn.70202
GSK-3β and mTOR Phosphorylation Mediate the Reversible Regulation of Hypomagnetic Field on Adult Neural Stem Cell Proliferation
Abstract
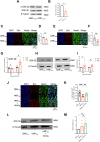
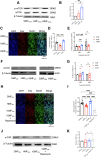
Exposure to hypomagnetic field (HMF) of < 5 μT has been demonstrated to impair cognitive behaviors in mammals by disrupting neurogenesis. This process could potentially be modulated by the protein phosphorylation of adult neural stem cells (aNSCs) that are highly sensitive to environmental changes. However, the effects of HMF on aNSCs protein phosphorylation remain unclear. Here, we found that HMF reversibly regulates the effects on aNSC proliferation by modulating protein phosphorylation in aNSCs. Specifically, HMF inhibits aNSCs proliferation by reducing glycogen synthase kinase 3β (GSK-3β) phosphorylation, and when aNSCs are returned from HMF to the geomagnetic field (rGMF), rGMF activates mammalian target of rapamycin (mTOR) phosphorylation to restore their proliferation. These findings not only advance our understanding of the molecular basis of HMF-induced biological effects but also illuminate potential therapeutic targets for maintaining neural homeostasis in extreme environments.
Keywords: GSK‐3β; adult neural stem cells; hypomagnetic field; mTOR; protein phosphorylation.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Osthole inhibits GSK-3β/AMPK/mTOR pathway-controlled glycolysis and increases radiosensitivity of subcutaneous transplanted hepatocellular carcinoma in nude mice.Strahlenther Onkol. 2024 May;200(5):444-452. doi: 10.1007/s00066-023-02173-8. Epub 2023 Nov 14. Strahlenther Onkol. 2024. PMID: 37963994
-
Essential role of p21Waf1/Cip1 in the modulation of post-traumatic hippocampal Neural Stem Cells response.Stem Cell Res Ther. 2024 Jul 6;15(1):197. doi: 10.1186/s13287-024-03787-0. Stem Cell Res Ther. 2024. PMID: 38971774 Free PMC article.
-
Decoding the Role of Neurotrophins in Glycogen Synthase Kinase 3-Beta Regulation in Alzheimer's Disease.Mol Neurobiol. 2025 Jul;62(7):8603-8623. doi: 10.1007/s12035-025-04776-x. Epub 2025 Feb 27. Mol Neurobiol. 2025. PMID: 40014269 Review.
-
Transcription Factor EB Overexpression through Glial Fibrillary Acidic Protein Promoter Disrupts Neuronal Lamination by Dysregulating Neurogenesis during Embryonic Development.Dev Neurosci. 2025;47(1):40-54. doi: 10.1159/000538656. Epub 2024 Apr 18. Dev Neurosci. 2025. PMID: 38583418 Free PMC article.
-
Could protein phosphatase 2A and glycogen synthase kinase-3 beta be targeted by natural compounds to ameliorate Alzheimer's pathologies?Brain Res. 2024 Apr 15;1829:148793. doi: 10.1016/j.brainres.2024.148793. Epub 2024 Feb 1. Brain Res. 2024. PMID: 38309553 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

