Weight reduction over time in tirzepatide-treated participants by early weight loss response: Post hoc analysis in SURMOUNT-1
- PMID: 40677091
- PMCID: PMC12326891
- DOI: 10.1111/dom.16554
Weight reduction over time in tirzepatide-treated participants by early weight loss response: Post hoc analysis in SURMOUNT-1
Abstract
Aims: The objective was to assess weight reduction at Weeks 24 and 72 in participants treated with tirzepatide based on weight reduction response after 12 weeks of treatment in the SURMOUNT-1 trial.
Materials and methods: This post hoc analysis included participants treated with tirzepatide who received ≥75% of the assigned treatment doses and had weight measurements at Weeks 0, 12, 24 and 72. Participants were categorized based on the 12-week response to tirzepatide: late responders (<5% weight reduction at Week 12) or early responders (≥5% weight reduction at Week 12).
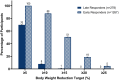
Results: A total of 1545 participants were included in the analyses, with 278 (18%) categorized as late responders and 1267 (82%) categorized as early responders. At baseline, late responders compared to early responders were more likely to be male (45% vs. 30%) and had higher body weight (110.2 vs. 103.6 kg), body mass index (BMI) (39.1 vs. 37.7 kg/m2) and waist circumference (117.5 vs. 113.4 cm). At the end of dose titration, Week 24, 194 (70%) late responders achieved ≥5% body weight reduction. At Week 72, 250 (90%) late responders achieved ≥5% body weight reduction. The mean time to reach 5% weight reduction for late responders was 24.8 ± 12.7 weeks. Higher doses of tirzepatide were associated with higher proportions of participants achieving various weight reduction thresholds at Weeks 24 and 72.
Conclusions: Among late responders to tirzepatide, the vast majority (90%) achieved 5% or more weight reduction at Week 72. This finding suggests that extending treatment well beyond 12 weeks may allow additional patients to achieve clinically meaningful weight reduction.
Trial registration: ClinicalTrials.gov, identifier: NCT04184622, available at http://www.
Clinicaltrials: gov/.
Keywords: response; tirzepatide.
© 2025 Eli Lilly and Company and The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Jamy Ard received research support from Nestle Healthcare Nutrition, Eli Lilly and Company, Boehringer Ingelheim, Epitomee, Inc., UnitedHealth Group R&D, KVKTech, WW and Novo Nordisk and is a consultant for Nestle Healthcare Nutrition, Eli Lilly and Company, Optum Labs R&D, Novo Nordisk, Intuitive, Regeneron, Brightseed, Amplifier Therapeutics, Amgen and Ingredion. Dr. Ard is an advisory board member for Novo Nordisk, Nestle Healthcare Nutrition, Eli Lilly and Company, WW and Boehringer Ingelheim. He is a member of the International Food Information Council—Assembly, The Obesity Society—President 2024, American Diabetes Association, Society of Behavioural Medicine, Roundtable on Obesity Solutions, American Society for Nutrition and American Society for Nutrition Foundation—Board of Trustees Executive Committee. Kimberly Gudzune was an Associate Professor in the Department of Medicine at the Johns Hopkins University School of Medicine and engaged in this research as a private advisor and not in her capacity as a Johns Hopkins faculty member. She received no compensation for this work. She has received personal fees for participation on advisory boards for Eli Lilly and Company and Novo Nordisk and travel support from Eli Lilly and Company and Novo Nordisk. Her former institution (Johns Hopkins) received grant funding from Novo Nordisk. Since the initial conduct of this research, Dr. Gudzune reports being an employee of the American Board of Obesity Medicine Foundation. Brandi Addison is a speaker for Eli Lilly and Company, Novo Nordisk and Corcept Therapeutics and is an advisory board member for Eli Lilly and Company. She is a member of the American Association of Clinical Endocrinology and The Obesity Society. Ildiko Lingvay received research funding (paid to institution) and/or products from Novo Nordisk, Sanofi, Boehringer‐Ingelheim, Dexcom and received research‐related consulting fees (paid to institution) from Novo Nordisk. She received advisory/consulting fees and/or other support from: AbbVie, Altimmune, Alveus Therapeutics, Amgen, Antag Therapeutics, AstraZeneca, Bayer, Betagenon AB, Bioio Inc., Biomea, Boehringer‐Ingelheim, Carmot, Cytoki Pharma, Eli Lilly and Company, Intercept, Janssen/J&J, Juvena, Keros Therapeutic, Inc., Mediflix, Merck, Metsera, Neurocrine, Novo Nordisk, Pharmaventures, Pfizer, Regeneron, Roche, Sanofi, Shionogi, Source Bio, Structure Therapeutics, TARGET RWE, TERNS Pharma, The Comm Group, WebMD and Zealand Pharma. Clare J. Lee, Dachuang Cao, Casey J. Mast, Adam Stefanski, Beverly Falcon and Donna Mojdami are employees and shareholders of Eli Lilly and Company.
Figures


References
-
- Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1‐203. - PubMed
-
- Jensen MD, Ryan DH, Donato KA, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults. Obesity. 2014;22(S2):S5‐S39. - PubMed
-
- Wilding JPH, Batterham RL, Calanna S, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. - PubMed

