A Membrane-Based Strategy for the High-Throughput Determination of Steroidal Hormones in Human Urine Using Natural Deep Eutectic Solvents Combined With Liquid Chromatography Coupled With Diode Array Detector
- PMID: 40677118
- PMCID: PMC12271989
- DOI: 10.1002/jssc.70229
A Membrane-Based Strategy for the High-Throughput Determination of Steroidal Hormones in Human Urine Using Natural Deep Eutectic Solvents Combined With Liquid Chromatography Coupled With Diode Array Detector
Abstract
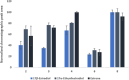
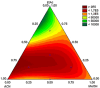
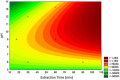
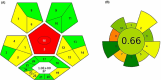
Estrogen hormones are present in the human body at varying concentrations, either naturally or through ingestion. At regulated levels, these compounds perform vital functions in the body. However, elevated concentrations are related to deregulation of several processes, such as abnormal cell proliferation, which can lead to the development of cancer (breast and ovarian) and other diseases (endometriosis). In this work, an analytical methodology is proposed for the determination of 17β-estradiol (E1), estrone (E2), and 17α-ethinylestradiol (EE2) in human urine. Hollow fiber-microporous membrane liquid-liquid extraction (HF-MMLLE) is used with natural deep eutectic solvents (NADESs). This technique was associated with a 96-well plate system followed by high-performance liquid chromatography coupled with a diode array detector (HPLC-DAD). The optimized conditions consisted of using thymol and camphor (1:1 v/v) as extraction solvent; an extraction time of 100 min; acetonitrile as desorption solvent; a desorption time of 30 min; and a sample pH adjusted to 12. Limits of quantification (LOQs) of 25 ng mL-1 for E2 and 50 ng mL-1 for E1 and EE2, and limits of detection (LOD) of 7.5 ng mL-1 for E2 and 15 ng mL-1 for E1 and EE2 were obtained. Intraday and interday precisions were lower than 8.7% and 21.6%, respectively. Relative recoveries were examined in two samples and ranged from 68.2% to 131.8%. The method was applied in samples from healthy volunteers, and concentrations from 75.6 to 182.7 ng mL-1 for E2 were found. Finally, the method was evaluated by two sustainable metrics to examine the green aspects of the experimental approach.
Keywords: bioanalytical methods; estrogens; microextraction; sustainability; urine monitoring.
© 2025 The Author(s). Journal of Separation Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Fast determination of per- and polyfluoroalkyl substances in human serum by cold-induced phase separation coupled with liquid chromatography-tandem mass spectrometry].Se Pu. 2025 Jul;43(7):756-766. doi: 10.3724/SP.J.1123.2024.11028. Se Pu. 2025. PMID: 40610770 Free PMC article. Chinese.
-
High throughput liquid phase thin film microextraction of estrogens by solvent-loaded polyvinylidene fluoride membrane sheet.J Chromatogr A. 2025 Sep 27;1759:466252. doi: 10.1016/j.chroma.2025.466252. Epub 2025 Jul 26. J Chromatogr A. 2025. PMID: 40768811
-
Deep eutectic solvent-based supramolecular solvent for dispersive liquid-liquid microextraction of aflatoxin B1 from safflower oil seeds prior to HPLC.Anal Methods. 2025 Jun 26;17(25):5269-5275. doi: 10.1039/d5ay00014a. Anal Methods. 2025. PMID: 40512597
-
Blood biomarkers for the non-invasive diagnosis of endometriosis.Cochrane Database Syst Rev. 2016 May 1;2016(5):CD012179. doi: 10.1002/14651858.CD012179. Cochrane Database Syst Rev. 2016. PMID: 27132058 Free PMC article.
-
Monitoring, sources, receptors, and control measures for three European Union watch list substances of emerging concern in receiving waters - A 20year systematic review.Sci Total Environ. 2017 Jan 1;574:1140-1163. doi: 10.1016/j.scitotenv.2016.09.084. Epub 2016 Oct 14. Sci Total Environ. 2017. PMID: 27741430
References
-
- Tomšíková H., Aufartová J., Solich P., Sosa‐Ferreira Z., Santana‐Rodríguez J. J., and Nováková L., “High‐Sensitivity Analysis of Female‐Steroid Hormones in Environmental Samples,” TrAC Trends in Analytical Chemistry 34 (2012): 35–58, 10.1016/j.trac.2011.11.008. - DOI
-
- do Carmo S. N., Merib J., and Carasek E., “Bract as a Novel Extraction Phase in Thin‐Film SPME Combined With 96‐Well Plate System for the High‐Throughput Determination of Estrogens in Human Urine by Liquid Chromatography Coupled to Fluorescence Detection,” Journal of Chromatography B 1118–1119 (2019): 17–24, 10.1016/j.jchromb.2019.04.037. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

