Artificial intelligence-driven computational methods for antibody design and optimization
- PMID: 40677216
- PMCID: PMC12279266
- DOI: 10.1080/19420862.2025.2528902
Artificial intelligence-driven computational methods for antibody design and optimization
Abstract
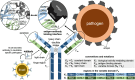
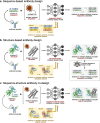
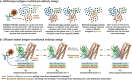
Antibodies play a crucial role in our immune system. Their ability to bind to and neutralize pathogens opens opportunities to develop antibodies for therapeutic and diagnostic use. Computational methods capable of designing antibodies for a target antigen can revolutionize drug discovery, reducing the time and cost required for drug development. Artificial intelligence (AI) methods have recently achieved remarkable advancements in the design of protein sequences and structures, including the ability to generate scaffolds for a given motif and binders for a specific target. These generative methods have been applied to antigen-conditioned antibody design, with experimental binding confirmed for de novo-designed antibodies. This review surveys current AI methods used in antibody development, focusing on those for antigen-conditioned antibody design. The results obtained by AI-based methodologies in antibody and protein research suggest a promising direction for generating de novo binders for various target antigens.
Keywords: Antibody design; generative artificial intelligence; machine learning; protein design; structural biology.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Chenoweth A, Crescioli S. Therapeutic monoclonal antibodies approved or in regulatory review. The Antibody Society. 2024. https://www.antibodysociety.org/antibody-therapeutics-product-data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
