Acupuncture treatment preserves soleus muscle mass and improves mitochondrial function in a rat model of disuse atrophy
- PMID: 40677295
- PMCID: PMC12269403
- DOI: 10.1016/j.imr.2025.101178
Acupuncture treatment preserves soleus muscle mass and improves mitochondrial function in a rat model of disuse atrophy
Abstract
Background: Muscle atrophy leads to debilitating loss of physical capacity, particularly when alternative treatments are needed. Acupuncture is proposed as a potential therapy for disuse atrophy, but its effects on muscle biology remain unclear. This study evaluated the effects of acupuncture on soleus muscle mass and mitochondrial function in a rat model of immobilization-induced atrophy.
Methods: Female Sprague Dawley rats were assigned to three groups: Control (CON), casting-induced immobilization (CT), and CT with acupuncture (CT-A) (n = 8). Immobilization of the left hindlimb lasted for 14 days, and acupuncture was performed at specific acupoints (stomach-36, gallbladder-34) three times per week for 15 min. Mitochondrial function was assessed in saponin-permeabilized fibers, and signaling molecules regulating muscle mass were analyzed by Western blot.
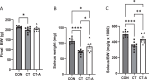
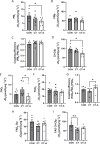
Results: CT-A attenuated soleus muscle atrophy compared to CT. Under fatty acid substrate conditions, CT reduced complex I and II-supported oxidative phosphorylation (OXPHOS) compared to CON, while CT-A decreased respiratory leak and enhanced OXPHOS coupling relative to CT. Without fatty acids, CT-A decreased both respiratory leak and complex I and II-supported OXPHOS compared to CON, but differences between CT and CT-A were not significant. AMPKα activity (p-AMPKα/AMPKα) was significantly elevated in the CT group compared to the CON group, but returned to CON levels in the CT-A group. However, there were no changes in proteins associated with muscle atrophy or autophagy markers among the groups.
Conclusion: Acupuncture mitigates immobilization-induced muscle atrophy and preserves mitochondrial function, suggesting its potential as a therapeutic approach for muscle disuse conditions.
Keywords: Acupuncture; Immobilization; Mitochondrial respiration; Muscle atrophy.
© 2025 Korea Institute of Oriental Medicine. Published by Elsevier B.V.
Figures


Similar articles
-
Acupuncture for the prevention of episodic migraine.Cochrane Database Syst Rev. 2016 Jun 28;2016(6):CD001218. doi: 10.1002/14651858.CD001218.pub3. Cochrane Database Syst Rev. 2016. PMID: 27351677 Free PMC article.
-
Acupuncture for acute hordeolum.Cochrane Database Syst Rev. 2017 Feb 9;2(2):CD011075. doi: 10.1002/14651858.CD011075.pub2. Cochrane Database Syst Rev. 2017. PMID: 28181687 Free PMC article.
-
Acupuncture for treating fibromyalgia.Cochrane Database Syst Rev. 2013 May 31;2013(5):CD007070. doi: 10.1002/14651858.CD007070.pub2. Cochrane Database Syst Rev. 2013. PMID: 23728665 Free PMC article.
-
Alternative Treatments to Exercise for the Attenuation of Disuse-Induced Skeletal Muscle Atrophy in Rats.Muscles. 2024 Jul 22;3(3):224-234. doi: 10.3390/muscles3030020. Muscles. 2024. PMID: 40757592 Free PMC article.
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
References
-
- Zhang H., Qi G., Wang K., Yang J., Shen Y., Yang X., et al. Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol. 2023;214 - PubMed
LinkOut - more resources
Full Text Sources

