Therapeutic Duel of Rifaximin Versus Lactulose in Hepatic Encephalopathy: A Systematic Review
- PMID: 40677427
- PMCID: PMC12268772
- DOI: 10.7759/cureus.86193
Therapeutic Duel of Rifaximin Versus Lactulose in Hepatic Encephalopathy: A Systematic Review
Abstract
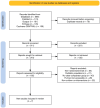
This systematic review aimed to compare the clinical efficacy of rifaximin versus lactulose in the management of hepatic encephalopathy (HE) by analyzing evidence from randomized controlled trials (RCTs). A comprehensive search across major databases identified seven eligible RCTs encompassing 693 adult patients diagnosed with overt or minimal HE. Findings demonstrated that rifaximin is at least as effective as lactulose in reversing HE symptoms, with some studies reporting significantly higher HE reversal rates when rifaximin was used in combination with lactulose (e.g., 76% vs. 50.8%, p<0.004), reduced mortality (23.8% vs. 49.1%, p<0.05), and shorter hospital stays (5.8 vs. 8.2 days, p=0.001). While other trials reported similar efficacy between the two agents (e.g., HE improvement: 84.4% vs. 95.4%, p=0.315), rifaximin was generally associated with better tolerability and fewer gastrointestinal side effects. These results support rifaximin as an effective and well-tolerated therapeutic option, either as monotherapy or in combination with lactulose. Further large-scale, multicenter trials are warranted to assess long-term outcomes, recurrence rates, and cost-effectiveness.
Keywords: cirrhosis; hepatic encephalopathy; lactulose; neurocognitive impairment; randomized controlled trial; rifaximin; treatment efficacy.
Copyright © 2025, Oriko et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources