Liver injury in post-acute COVID-19 syndrome: A systematic review and meta-analysis of early observational studies
- PMID: 40677535
- PMCID: PMC12269275
- DOI: 10.3138/canlivj-2024-0010
Liver injury in post-acute COVID-19 syndrome: A systematic review and meta-analysis of early observational studies
Abstract
Background: Post-acute COVID-19 syndrome (PACS; long COVID) is characterized by persistent or delayed symptoms at least 4 weeks from acute COVID-19 infection. Given the well-documented incidence of liver injury in acute COVID-19, this systematic review aims to assess the odds of liver injury in earlier experiencers of PACS.
Methods: Observational studies published prior to March 2022 were screened for data describing liver injury (defined per primary study) in patients with PACS.
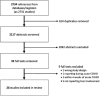
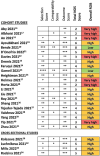
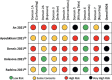
Results: A total of 2,117 abstracts and 35 full texts were screened, of which 26 met the inclusion criteria. The mean time since acute COVID infection across all studies was 195.5 days. Seven studies included COVID-negative control groups. Twenty-three studies measured lab findings, and nine studies measured imaging or elastography. Five studies were eligible for meta-analysis of odds ratios, which did not demonstrate a statistically significant difference in odds for liver injury in patients with PACS compared with COVID-negative patients (OR 2.22 [95% CI 0.51-9.61; p = 0.28]). Newcastle-Ottawa Scale assessments for all studies found 24 of 26 studies with high to very high risk of bias. ROBINS-E assessments for studies included in the meta-analysis found five of five studies with high to very high risk of bias.
Conclusions: Overall, our findings demonstrate no statistical difference in odds ratios of liver injury in patients with PACS compared with COVID-negative controls. As such, routine assessment and monitoring of liver injury in patients with PACS may not be required; however, higher quality data with lower risk of bias are required to make recommendations of higher certainty.
Keywords: COVID-19; PACS; hepatology; liver injury; long COVID; post-acute COVID-19 syndrome.
Plain language summary
Lay Summary: Post-acute COVID-19 syndrome (PACS), often called long COVID, refers to lingering symptoms following recovery from acute COVID-19 infection. Liver injury is a documented complication of acute COVID-19, but its presence in patients with PACS has not been clarified. We systematically searched the literature and examined 26 studies reporting on liver injury in patients with PACS. We found that although abnormalities in liver function tests and imaging were observed in some studies, there was not a significant increase in liver injury in patients with PACS compared with COVID-negative individuals. This suggests that monitoring patients with PACS for liver injury may not be necessary and thus may save the health care system significant time and money. However, the quality of evidence was very limited, with many studies showing biases and disparities, and so, we are unable to make concrete recommendations. More research with better quality data is needed to confirm our suggestions. Future studies should focus on standardizing reporting, assessing the impact of vaccination, and investigating the effects of newer COVID-19 variants on liver health. Until then, routine liver testing in patients with PACS may not be warranted.
© Canadian Association for the Study of the Liver, 2024.
Conflict of interest statement
N/A
Figures

References
-
- Chippa V, Aleem A, Anjum F. Post-Acute Coronavirus (COVID-19) Syndrome - StatPearls - NCBI Bookshelf. - PubMed
-
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data.
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
