C/EBPβ-VCAM1 axis in Kupffer cells promotes hepatic inflammation in MASLD
- PMID: 40677688
- PMCID: PMC12270669
- DOI: 10.1016/j.jhepr.2025.101418
C/EBPβ-VCAM1 axis in Kupffer cells promotes hepatic inflammation in MASLD
Abstract
Background & aims: Kupffer cells (KCs) can promote hepatic inflammation in metabolic dysfunction-associated steatotic liver disease (MASLD), but the underlying molecular mechanisms are not fully understood. C/EBPβ in macrophages can mediate metabolic and immune dysregulations. Therefore, we aimed to explore its role in KCs in MASLD pathogenesis.
Methods: A 12-week high-fat and high-cholesterol diet (HFHCD) model was used in wild-type or KC-specific Cebpb heterozygous knockout mice (n = 10 per group), followed by liver evaluation using histopathology, flow cytometry, and RNA-seq. RNA-seq of liver tissue (n = 3 per group) and C/EBPβ CUT&Tag-seq of sorted KCs were comprehensively analyzed to elucidate the transcriptional regulatory network. Flow cytometry and immunofluorescence were used to detect the expression or distribution of key proteins.
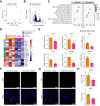
Results: HFHCD induced prominent immune cell infiltration and a concomitant increase in C/EBPβ in KCs. KC-specific Cebpb heterozygous knockout significantly reduced HFHCD-induced lobular inflammation (p <0.05) and inflammation-related gene expression (p <0.05) in the liver. Multi-omics analysis revealed increased C/EBPβ activity in KCs in MASLD, leading to a selective promotive effect on MASLD-induced genes. Further integrated analysis identified Vcam1 as a key direct downstream gene of C/EBPβ in KCs in MASLD, which involves C/EBPβ-mediated activation of the Vcam1 promoter. VCAM1 was predominantly expressed in KCs in the hepatic tissue of MASLD mice and patients. KC-expressed VCAM1 was significantly increased in MASLD compared with healthy controls (p <0.01), and it promoted immune cell infiltration into the liver.
Conclusions: Increased C/EBPβ in KCs promotes pathogenic transcriptional activation, leading to increased VCAM1 expression and inflammatory cell infiltration in MASLD. Inhibition of C/EBPβ in KCs might be a potential therapeutic strategy against hepatic inflammation in MASLD.
Impact and implications: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease worldwide, but its pathogenesis remains elusive. In this study, we investigated the critical role of CCAAT/enhancer binding protein β (C/EBPβ) in Kupffer cells and its implications in MASLD pathogenesis. We found that an increased C/EBPβ level in Kupffer cells promotes hepatic inflammation in MASLD by upregulating VCAM1 expression. Our findings provide valuable insights into the molecular mechanisms driving MASLD and propose a potential novel therapeutic target to mitigate hepatic inflammation in MASLD.
Keywords: C/EBPβ; Kupffer cell; MASH; MASLD; VCAM1.
© 2025 The Author(s).
Conflict of interest statement
The authors have no conflicts to report. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







References
-
- Rinella M.E., Lazarus J.V., Ratziu V., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–1556. - PubMed
-
- Targher G., Byrne C.D., Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691–702. - PubMed
-
- Schuster S., Cabrera D., Arrese M., et al. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–364. - PubMed
-
- Mladenić K., Lenartić M., Marinović S., et al. The “Domino effect” in MASLD: the inflammatory cascade of steatohepatitis. Eur J Immunol. 2024;54 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

