Mapping the hemodynamic effects of terlipressin in patients with hepatorenal syndrome using advanced magnetic resonance imaging
- PMID: 40677691
- PMCID: PMC12270624
- DOI: 10.1016/j.jhepr.2025.101452
Mapping the hemodynamic effects of terlipressin in patients with hepatorenal syndrome using advanced magnetic resonance imaging
Abstract
Background & aims: Terlipressin improves renal function in ∼40% of patients with hepatorenal syndrome-acute kidney injury (HRS-AKI). Nonetheless, the pathophysiological mechanisms of terlipressin remain unclear. Therefore, we investigated the cardiovascular changes that occur after terlipressin is given to patients with HRS-AKI.
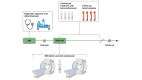
Methods: Cardiac and phase-contrast magnetic resonance imaging were used to assess cardiac function, as well as renal, splanchnic, and peripheral blood flow changes after the first bolus of 2 mg terlipressin in 10 patients with HRS-AKI, six of whom also had acute-on-chronic liver failure. Hemodynamic changes were analyzed using the Wilcoxon matched-pairs signed-rank test. Patients were followed prospectively to investigate any associations between terlipressin-induced hemodynamic changes and clinical outcomes.
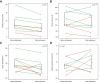
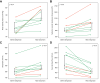
Results: Cardiac output (CO) decreased by 15% following terlipressin (p <0.01). Despite this decrease in CO, renal artery blood flow increased by 23% (p <0.01), and the renal artery blood flow percentage of CO increased by 49% (p = 0.01). Superior mesenteric artery blood flow and femoral artery blood flow decreased by 27% and 40%, respectively (both p <0.01). Mean arterial pressure (MAP) and systemic vascular resistance increased by 13% and 32%, respectively (both p <0.01). Baseline renal artery blood flow correlated with serum creatinine (p <0.01). By contrast, changes in renal artery blood flow and other cardiocirculatory variables did not correlate with changes in serum creatinine after terlipressin or with mortality.
Conclusions: Terlipressin increases renal artery blood flow, reduces CO, and alleviates splanchnic and peripheral vasodilatation. These effects, combined with an increase in MAP, appear to explain the therapeutic benefits of terlipressin in patients with HRS-AKI.
Impact and implications: This study is the first to provide a detailed mapping of the hemodynamic changes following terlipressin treatment in patients critically ill with HRS-AKI. The results indicate that the beneficial effects of terlipressin are driven by selective peripheral and splanchnic vasoconstriction, which redistributes blood flow, normalizes MAP, and ultimately improves renal perfusion despite reduced cardiac output. This study also highlights the advantages of using magnetic resonance imaging as a non-invasive method to evaluate pharmacological interventions, with the potential to contribute to future advances in personalized medicine for patients with cirrhosis.
Clinical trials registration: NCT03483272.
Keywords: Acute kidney injury; Acute-on-chronic liver failure; Pharmacodynamic; Portal hypertension; Terlipressin.
© 2025 The Authors.
Conflict of interest statement
The study was supported by an independent research grant from Ferring Pharmaceuticals A/S. HRS has received honoraria as speaker and consultant from Lundbeck AS, and as editor (of Neuroimage Clinical) from Elsevier. He has received royalties as a book editor from Springer, Oxford University Press, and Gyldendal. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Wong F., Pappas S.C., Curry M.P., et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–828. - PubMed
-
- Ginès P., Solà E., Angeli P., et al. Hepatorenal syndrome. Nat Rev Dis Prim. 2018;4:23. - PubMed
-
- European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. - PubMed
-
- Belcher J.M., Parada X.V., Simonetto D.A., et al. Terlipressin and the treatment of hepatorenal syndrome: how the CONFIRM trial moves the story forward. Am J Kidney Dis. 2022;79:737–745. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

