Transjugular diagnostic procedures in hepatology: Indications, techniques and interpretation
- PMID: 40677692
- PMCID: PMC12269627
- DOI: 10.1016/j.jhepr.2025.101437
Transjugular diagnostic procedures in hepatology: Indications, techniques and interpretation
Abstract
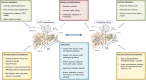
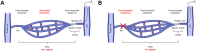
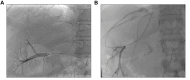
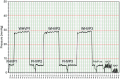
Measurement of the hepatic venous pressure gradient (HVPG) and transjugular liver biopsy have emerged as important tools in clinical hepatology. Measurement of HVPG is considered the gold standard for detecting clinically significant portal hypertension, with an HVPG of ≥10 mmHg being the key prognostic threshold in patients with compensated advanced chronic liver disease (cACLD; compensated cirrhosis). A transjugular liver biopsy can be obtained within the same procedure and may be preferred over percutaneous liver biopsy in patients with coagulopathy, ascites and/or significant obesity. Endoscopic ultrasound-guided procedures are currently under investigation and require standardisation. This article summarises critical technical aspects of HVPG measurements and transjugular liver biopsy and provides a detailed overview of their current role in the context of emerging non-invasive tests and endoscopic approaches.
Keywords: Cirrhosis; HVPG; PSVD; hepatic venous pressure gradient; minimally invasive; portal hypertension; porto-sinusoidal vascular disorder; transjugular liver biopsy.
© 2025 The Author(s).
Conflict of interest statement
DB: Lecture fees/consulting: W. L. Gore & Associates GmbH, Travel grant: Gilead Science. AB: Consulting: Boehringer-Ingelheim; lecture fees: W. L. Gore & Associates GmbH; GE Healthcare; Hologic. MM: Speaker and/or consultant and/or advisory board member for AbbVie, Collective Acumen, Gilead, Echosens, Ipsen, Takeda, and W. L. Gore & Associates; travel support from AbbVie and Gilead. CR: Consulting: Boehring-Ingelheim, lecture fees: W. L. Gore & Associates GmbH, Falk Foundation, Bristol-Myers Squibb. EZ: lecture fees: Abbvie, Gilead, Dr. Falk Pharma; travel grants: Abbvie, Gilead, W.L. Gore& Associates; advisory: Bentley InnoMed. TB: received consulting fees from Intercept/Advanz Pharma, Grifols, and Sobi; honoraria for lectures, presentations, or educational events from Falk Foundation, CSL Behring, Merck, Gilead, Intercept/Advanz Pharma, and Gore; travel support from Gilead. CE: Advisory: Boehringer-Ingelheim, Albireo, Lecture fees: Albireo, Gilead. VF: Advisory: Astra Zeneca, ADVITOS, Lecture fees: CSL-Behring, ADVITOS, Astra Zeneca, Merz. PAR: lecture fees: Pfizer, BMS, CSL Behring, CSL Seqirus, AstraZeneca; Advisory Board: Gilead, ADVANZ, Pfizer, travel support AbbVie, Ipsen. MS: lecture fees/consulting: Falk Foundation e.V., W. L. Gore & Associates, Bentley InnoMed GmbH. AZ: Lecture fees/consulting: CLS Behringer, Lecture fees: W. L. Gore & Associates GmbH, Falk Foundation, CML: consulting fees from Abbvie, AstraZeneca, Boston Scientific, CSL Behring, Eisai, Falk, Gilead, Norgine, Roche, Shionogi, and Sobi; honoraria for lectures, presentations, or educational events from Abbvie, AstraZeneca, Boston Scientific, CSL Behring, Gore, Eisai, Falk, and Norgine. TR: received grant support from Abbvie, Boehringer Ingelheim, Gilead, Intercept/Advanz Pharma, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, Siemens and W. L. Gore & Associates; speaking honoraria from Abbvie, Gilead, Intercept/Advanz Pharma, Roche, MSD, W. L. Gore & Associates; consulting/advisory board fee from Abbvie, Astra Zeneca, Bayer, Boehringer Ingelheim, Gilead, Intercept/Advanz Pharma, MSD, Resolution Therapeutics, Siemens; and travel support from Abbvie, Boehringer Ingelheim, Dr. Falk Pharma, Gilead and Roche. RK: received consulting fees from Boston Scientific, Bristol Myers Squibb, Guerbet, Roche, and Sirtex and lecture fees from Astra Zeneca, BTG, Eisai, Guerbet, Ipsen, Roche, Siemens, Sirtex, MSD Sharp & Dohme and is on the data safety monitoring board of the ABC HCC Trial. Furthermore, he serves as the Chair of the Audit and Standards Subcommittee of the European Society of Radiology. All of these roles are not related to this project. JB: Consultant to AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Resolution Therapeutics. MD: Lecture fees/consulting: Sanofi-Aventis, Ipsen Pharma, Allpha Sigma Pharma, Takeda Pharmaceuticals, Falk Foundation, Mainz Biomed GmbH; Travel grant: Gilead Science, Falk Foundation. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Clinical practice guidelines of the European association for the study of the liver - advancing methodology but preserving practicability. J Hepatol. 2019;70:5–7. - PubMed
-
- Steinlauf A.F., Garcia-Tsao G., Zakko M.F., et al. Low-dose midazolam sedation: an option for patients undergoing serial hepatic venous pressure measurements. Hepatol Baltim Md. 1999;29:1070–1073. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

