Phosphatidylethanol and ethyl glucuronide to categorize alcohol consumption in alcohol-related cirrhosis
- PMID: 40677698
- PMCID: PMC12269596
- DOI: 10.1016/j.jhepr.2025.101433
Phosphatidylethanol and ethyl glucuronide to categorize alcohol consumption in alcohol-related cirrhosis
Abstract
Background & aims: Phosphatidylethanol (PEth) is an alcohol-use biomarker that could bridge the detection windows of urinary ethyl glucuronide (uEtG) and scalp hair ethyl glucuronide (hEtG), but has been rarely validated in patients with liver disease. Reported detection windows of these biomarkers also vary significantly, and available studies have focused solely on any alcohol use. Yet, categorizing patients with liver disease based on their level of alcohol use would be highly informative. Here, we assessed the diagnostic accuracy and optimal detection windows of whole-blood PEth, uEtG, hEtG, and the novel biomarker fingernail ethyl glucuronide (nEtG), for different levels of alcohol use in patients with alcohol-related cirrhosis.
Methods: Patients with alcohol-related cirrhosis were questioned on their alcohol use over the previous 3 months using the Alcohol Timeline Followback (n = 116). In addition, 1-7-day (uEtG), 1-5-week (PEth), and 3-month (hEtG and nEtG) detection windows were assessed for any, increased (women ≥2 units/day or men ≥3 units/day), or excessive alcohol use (women ≥5 units/day or men ≥6 units/day).
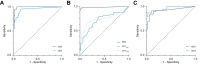
Results: uEtG, PEth, and hEtG had high diagnostic accuracies for any alcohol use at optimal detection windows of 3 days [area under the receiver operating characteristic curve (AUROC): 0.990 (95% confidence interval (CI): 0.975-1.000)], 3 weeks [AUROC: 0.986 (95% CI: 0.958-1.000)], and 3 months [AUROC: 0.925 (95% CI: 0.862-0.987)], respectively. They had high negative predictive values (>92%) for increased and excessive use. nEtG showed promising results for assessing any alcohol use over the previous 3 months [AUROC: 0.962 (95% CI: 0.924-1.000)].
Conclusions: PEth and EtG have excellent and complementary diagnostic accuracies to detect any alcohol use and rule out increased alcohol use in patients with alcohol-related cirrhosis. nEtG provides an alternative for hEtG, but requires further validation.
Impact and implications: The correct identification and categorization of alcohol use is a major challenge in the treatment of patients with liver disease. Furthermore, given the new nomenclature toward steatotic liver disease, it has become essential to be able to categorize alcohol use into any, increased, or excessive use. The validation of PEth and urine, scalp hair, and nail EtG in patients with alcohol-related liver disease provides us with reliable options to overcome these issues in both clinical care and pharmacological trials on steatotic liver disease.
Clinical trials registration: The study is registered at ClinicalTrials.gov (NCT04363424).
Keywords: Alcohol drinking; Alcoholic cirrhosis; Alcoholic liver diseases; Ethyl glucuronide; Metabolic dysfunction-associated steatotic liver disease; Phosphatidylethanol.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. - PubMed
-
- Staufer K., Huber-Schönauer U., Strebinger G., et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77:918–930. - PubMed
-
- Díaz L.A., Arab J.P., Louvet A. et al.The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20:764–783. - PubMed
-
- Andresen-Streichert H., Beres Y., Weinmann W., et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: results of a prospective study. Transpl Int. 2017;30:611–620. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

