Associations between night/shift working and late-life brain health
- PMID: 40677699
- PMCID: PMC12268499
- DOI: 10.1093/braincomms/fcaf264
Associations between night/shift working and late-life brain health
Abstract
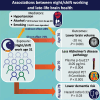
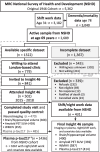
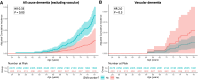
Sleep and circadian disturbances are associated with increased dementia risk. The mechanism remains poorly understood. We aimed to examine the relationship between night/shift working at age 31 and biomarkers of late-life brain health and to estimate the extent to which these relationships are mediated by unhealthy lifestyle behaviours. A prospective longitudinal cohort study, Insight 46, recruited participants from the Medical Research Council National Survey of Health and Development (NSHD) 1946 British Birth cohort. All born in the same week in 1946, participants were assessed at age 70 with multi-modal structural and molecular brain imaging and fluid biomarkers, from which whole-brain and hippocampal volumes, white matter hyper-intensity volume (WMHV), 18F-florbetapir amyloid-β PET Centiloids and plasma phosphorylated tau (p-tau)217 were derived. Prospective data collection included night/shift working at age 31, alongside smoking, alcohol intake, body mass index, exercise, blood pressure, Framingham risk score (FRS) at multiple timepoints from age 20 to 70 and dementia diagnosis or death by age 78. Analyses were adjusted for sex, age, education, socioeconomic position and, where appropriate, total intracranial volume or apolipoprotein E (APOE) genotype. Night/shift working data were available for 431 Insight 46 participants {50% female, mean age 70.7 years [standard deviation (SD) 0.6]}. Night/shift workers had lower whole-brain volume [-19.9 mL, 95% confidence interval (CI) -31.9, -7.9, P = 0.001], lower amyloid PET Centiloids (-9.45, 95% CI -14.7, -4.1, P = 0.0008) and lower plasma p-tau217 concentration (-0.05 pg/mL, 95% CI -0.10, -0.001, P = 0.04), without significant difference in hippocampal volume or WMHV. p-tau217 concentrations were also lower in night/shift workers from a wider sample from the NSHD cohort [n = 1067, mean age 69.9 (SD 0.7), -0.05 pg/mL, 95% CI -0.08, -0.02, P = 0.004]. By age 78, night/shift workers in the NSHD cohort (n = 3040) had lower rates of all-cause (excluding vascular) dementia (hazard ratio 0.33, 95% CI 0.12, 0.92, P = 0.03). Night/shift workers had 0.6% higher FRS (P = 0.01) at age 36, smoked 5.9 more pack-years by age 53 (P = 0.005), consumed 10.7 g/day more alcohol by age 63 (P = 0.006) and had higher rates of APOE ɛ4 allele carriage. Lifestyle behaviours mediated 28% of the lower brain volume in night/shift workers. Despite less healthy lifestyles, higher rates of APOE ɛ4 allele carriage and smaller brains, night/shift workers had lower levels of Alzheimer's disease pathology as measured by amyloid PET and plasma p-tau217 and approximately one-third of the risk of dementia by age 78 compared with non-night/shift workers. Lower brain volume in night/shift workers was partially mediated by unhealthy behaviours. Reduced dementia risk in night/shift workers is unexpected and will require further study.
Keywords: Alzheimer’s disease; circadian rhythms; dementia; shift work; sleep.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
A.K. is an executive member of the Biofluid Biomarkers Professional Interest Area of the Alzheimer’s Association (unpaid). D.M.C. is the chair of the Neuroimaging Professional Interest Area of the Alzheimer’s Association (unpaid). J.M.S. has received research funding and PET tracer from AVID Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) and Alliance Medical; has consulted for Roche, Eli Lilly, Biogen, AVID, Merck and GE; and received royalties from Oxford University Press and Henry Stewart Talks. He is a Chief Medical Officer for Alzheimer’s Research UK.
Figures



References
-
- Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
