Oncostatin M enhances the lengthening of sensory nerves and skin hypersensitivity
- PMID: 40677708
- PMCID: PMC12267034
- DOI: 10.3389/fimmu.2025.1571120
Oncostatin M enhances the lengthening of sensory nerves and skin hypersensitivity
Abstract
Background: Oncostatin M (OSM) is a cytokine that mediates inflammatory processes and is overexpressed in skin lesions of atopic dermatitis (AD). By amplifying neural responses to chemicals such as histamine, OSM increases sensitivity to pruritus. However, the morphological effects of OSM on peripheral sensory nerves and their subsequent impact on pruritus remain unclear. This study investigated OSM-induced peripheral nerve elongation, which may contribute to skin hypersensitivity.
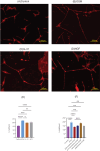
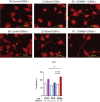
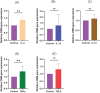
Methods: We assessed neurite outgrowth using primary mouse dorsal root ganglion (DRG) cells treated with OSM, IL-31, or nerve growth factor. Next, we pre-treated the cells with inhibitors of downstream signaling pathways of OSM, including extracellular signal-regulated kinase (ERK), signal transducers and activator of transcription (STAT) 3, c-Jun N-terminal kinase (JNK), and p38, followed by OSM administration to measure neurite outgrowth. Furthermore, OSM receptor β-overexpressing cell lines were established by gene transfer into the DRG cell line, and nerve elongation was measured after OSM administration. In vivo studies involved OSM administration in mouse skin models. Immunofluorescence staining was used to evaluate nerve elongation. We examined whether OSM-infused mice had increased hypersensitivity to mechanical stimuli-induced pruritus. Various cytokine stimuli were applied to CD4+ T cells isolated from healthy humans to examine the conditions under which OSM production increases.
Results: OSM significantly induced neurite outgrowth in DRG cells and the effect of OSM surpassed the effects of IL-31 and nerve growth factor. The neurite outgrowth effect of OSM involved the JAK/STAT3, MEK/ERK, and p38/MAPK pathways. Compared to control cells, DRG cell lines that overexpressed OSM receptor β showed significantly enhanced neurite outgrowth upon OSM treatment. In vivo, OSM treatment increased nerve elongation in the mouse dermis. Behavioral assays in mice showed that OSM administration increased sensitivity to mechanical stimuli. IL-4 and TNFα increased OSM production in CD4+ T cells.
Conclusion: OSM induces neurite elongation and may contribute to skin hypersensitivity. This suggests the potential utilization of OSM as a therapeutic target for inflammatory skin diseases such as AD.
Keywords: atopic dermatitis; itch; nerve elongation; oncostatin M; pruritus; sensory nerve.
Copyright © 2025 Ishikawa, Saito, Suehiro, Ishii, Yanase, Kawaguchi, Uchida, Yanagida, Numata, Sasaki, Kamigaki, Takeno and Tanaka.
Conflict of interest statement
AT has received speaker honoraria from Eli Lilly, Kaken Pharmaceutical, Sanofi, Taiho Pharmaceutical, AbbVie, Pfizer, Kyorin Pharmaceutical, Mitsubishi Tanabe, Torii Pharmaceutical, and Maruho, and a research grant from Maruho. ST has received speaker honoraria from Sanofi and Tanabe Mitsubishi Pharmaceutical Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Oncostatin M suppresses IL31RA expression in dorsal root ganglia and interleukin-31-induced itching.Front Immunol. 2023 Nov 16;14:1251031. doi: 10.3389/fimmu.2023.1251031. eCollection 2023. Front Immunol. 2023. PMID: 38035099 Free PMC article.
-
Toll-Like receptor 3-mediated interferon-β production is suppressed by oncostatin m and a broader epithelial-mesenchymal transition program.Breast Cancer Res. 2024 Nov 26;26(1):167. doi: 10.1186/s13058-024-01918-2. Breast Cancer Res. 2024. PMID: 39593161 Free PMC article.
-
Dominant dystrophic epidermolysis bullosa is associated with glycolytically active GATA3+ T helper 2 cells which may contribute to pruritus in lesional skin.Br J Dermatol. 2024 Jul 16;191(2):252-260. doi: 10.1093/bjd/ljae110. Br J Dermatol. 2024. PMID: 38477474
-
Topical tacrolimus for atopic dermatitis.Cochrane Database Syst Rev. 2015 Jul 1;2015(7):CD009864. doi: 10.1002/14651858.CD009864.pub2. Cochrane Database Syst Rev. 2015. PMID: 26132597 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Tominaga M, Takamori K. Recent advances in pathophysiological mechanisms of itch. Expert Rev Dermatol. (2010) 5:197–212. doi: 10.1586/edm.10.7 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

