From mechanism to application: programmed cell death pathways in nanomedicine-driven cancer therapies
- PMID: 40677757
- PMCID: PMC12270071
- DOI: 10.1016/j.bioactmat.2025.06.052
From mechanism to application: programmed cell death pathways in nanomedicine-driven cancer therapies
Abstract
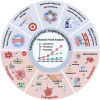
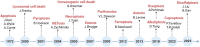
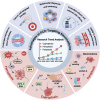
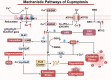
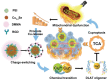
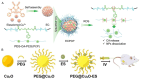
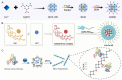
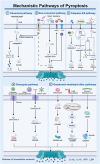
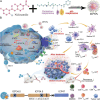
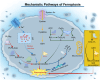
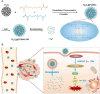
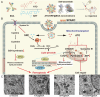
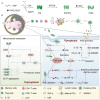
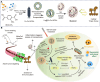
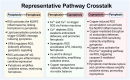
Programmed cell death (PCD) plays a crucial role in preventing cancer initiation and progression. Among the diverse PCD pathways, cuproptosis, pyroptosis, and ferroptosis have garnered attention for their unique mechanisms, which not only directly eliminate tumor cells but also enhance anti-tumor immunity. However, the therapeutic efficacy of PCD inducers is often compromised by rapid compensatory pathways in tumor cells, accelerated drug metabolism, and a lack of specificity, which can result in severe side effects. Engineered nanomedicines offer distinct advantages by leveraging nanoscale physicochemical properties to optimize pharmacokinetics, efficacy, and safety in cancer therapy. These nanomedicines enable precise targeting of tumor cells while enhancing drug stability. Moreover, they can simultaneously activate multiple PCD pathways and integrate with conventional therapies to further amplify anti-tumor effects. This review systematically examines the pathophysiological roles, mechanisms, and therapeutic implications of cuproptosis, pyroptosis, and ferroptosis in cancer treatment, with an emphasis on their modulation by nanomedicines. It also explores the potential interactions among these PCD pathways and highlights recent advancements in nanomedicine-based combination therapies targeting multiple PCD mechanisms. Finally, the challenges, limitations, and prospects for the clinical translation and application of PCD-targeting nanomedicines are discussed.
Keywords: Biomaterial; Cuproptosis; Ferroptosis; Nanomedicine; Programmed cell death; Pyroptosis.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

