Identification and Functional Characterization of a Novel PRPS1 Variant in X-Linked Nonsyndromic Hearing Loss: Insights From Zebrafish and Cellular Models
- PMID: 40677922
- PMCID: PMC12267977
- DOI: 10.1155/humu/6690588
Identification and Functional Characterization of a Novel PRPS1 Variant in X-Linked Nonsyndromic Hearing Loss: Insights From Zebrafish and Cellular Models
Abstract
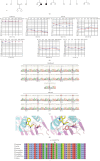
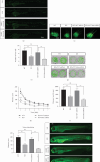
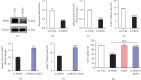
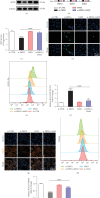
Purpose: The study was aimed at identifying the pathogenic gene responsible for X-linked nonsyndromic hearing loss (NSHL) in a five-generation Chinese family and at elucidating the gene's function both in vivo using a zebrafish model and in vitro using PRPS1 knockdown HEI-OC1 cells. Methods: Exome sequencing (ES) and Sanger sequencing were used to identify the pathogenic variants. A transgenic zebrafish model overexpressing the novel PRPS1 variant (c.494G>A: p.Cys165Tyr) was constructed, and PRPS1 was knocked down in HEI-OC1 cells using siRNA to explore the underlying mechanisms. Hair cell development and behavior were assessed in zebrafish, and mitochondrial function and cell viability were analyzed in HEI-OC1 cells. Results: A novel missense variant (c.494G>A: p.Cys165Tyr) in the PRPS1 gene was identified as the pathogenic variant causing progressive X-linked deafness-1 (DFNX1). The variant led to hair cell death in zebrafish, with disrupted swimming behavior. In HEI-OC1 cells, PRPS1 knockdown resulted in downregulation of the nicotinamide adenine dinucleotide (NAD+)/sirtuin 3 (SIRT3)/superoxide dismutase 2 (SOD2) pathway, increased reactive oxygen species (ROS) accumulation, mitochondrial dysfunction, and apoptosis, which were partially rescued by pretreatment with nicotinamide mononucleotide (NMN), a precursor of NAD+. Conclusion: The study reports a novel PRPS1 variant contributing to the variant spectrum of PRPS1 and highlights the role of PRPS1 deficiency in increasing oxidative stress-induced hair cell apoptosis via the NAD+/SIRT3/SOD2 pathway. These findings provide new insights into the molecular mechanisms of PRPS1-related hearing loss and potential therapeutic targets.
Keywords: PRPS1; X-linked nonsyndromic hearing loss; exome sequencing; novel variant; oxidative stress.
Copyright © 2025 Yining Wan et al. Human Mutation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Identification of IDH3G, encoding the gamma subunit of mitochondrial isocitrate dehydrogenase, as a novel candidate gene for X-linked retinitis pigmentosa.Genet Med. 2025 Jun;27(6):101418. doi: 10.1016/j.gim.2025.101418. Epub 2025 Mar 19. Genet Med. 2025. PMID: 40119724
-
Variants in KLF4 affecting residue Asp441 cause an autosomal dominant syndromic ichthyosis.Br J Dermatol. 2025 Jun 20;193(1):136-146. doi: 10.1093/bjd/ljaf062. Br J Dermatol. 2025. PMID: 39969530
-
Bioinformatics and experiments reveal the hub genes of age-related hearing loss and the mechanism of PLK1 silencing in the protection of aging cochlear hair cells.Gene. 2025 Sep 5;963:149632. doi: 10.1016/j.gene.2025.149632. Epub 2025 Jun 12. Gene. 2025. PMID: 40516835
-
Epidemiology, etiology, genetic variants in non- syndromic hearing loss in Iran: A systematic review and meta-analysis.Int J Pediatr Otorhinolaryngol. 2023 May;168:111512. doi: 10.1016/j.ijporl.2023.111512. Epub 2023 Mar 29. Int J Pediatr Otorhinolaryngol. 2023. PMID: 37086676
-
Genetic Homogeneity of a TDP1 Variant, c.1478A>G, as the Main Disease-Causing Variant of Spinocerebellar Ataxia With Axonal Neuropathy 1 (SCAN1) in the Middle East: A Systematic Review.Pediatr Neurol. 2025 Mar;164:41-52. doi: 10.1016/j.pediatrneurol.2024.12.011. Epub 2024 Dec 30. Pediatr Neurol. 2025. PMID: 39848142
References
-
- Hove-Jensen B., Andersen K. R., Kilstrup M., Martinussen J., Switzer R. L., Willemoës M. Phosphoribosyl diphosphate (PRPP): biosynthesis, enzymology, utilization, and metabolic significance. Microbiology and Molecular Biology Reviews . 2017;81(1):10–128. doi: 10.1128/MMBR.00040-16. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

