Management of non-traumatic abdominal pain in the emergency department: a multicentre, stepped-wedge, cluster-randomised trial
- PMID: 40678036
- PMCID: PMC12268095
- DOI: 10.1016/j.lanepe.2025.101362
Management of non-traumatic abdominal pain in the emergency department: a multicentre, stepped-wedge, cluster-randomised trial
Abstract
Background: Even though abdominal pain is one of the most frequent chief complaints in emergency medicine, standardized care pathways are still lacking. In this study, a standardized, digitally-supported care pathway for non-traumatic abdominal pain in the emergency department was investigated with regard to emergency department length of treatment, pain score at discharge, and patient satisfaction.
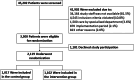
Methods: In a prospective mixed-methods, multicentre, cluster-randomised, controlled stepped wedge trial, adult patients with non-traumatic abdominal pain were enrolled in ten emergency departments across Germany. The new care pathway was implemented at randomly assigned time points (every four months) within a 24-month recruitment period and consisted of a structured care pathway for the management of abdominal pain patients in the emergency department. During the control period, the standard treatment for abdominal pain in the emergency department was administered. The planned sample size was 2000. Primary outcomes were: emergency department length of treatment, pain score (numeric rating scale 0-10), and patient satisfaction score at the end of emergency department treatment. Exploratory safety outcomes were serious adverse events within 30 days. Trial registration: DRKS00021052.
Findings: Of 2119 patients, 1017 were enrolled in the control group, and 1102 in the intervention group. Crude mean emergency department length of treatment was 5.2 h (±3.0) in the control group, and 4.3 h (±2.2) in the intervention group while the adjusted mean difference was -0.31 h (95% confidence interval (CI) -0.70 to 0.07). Mean pain score was 4.3 (±2.5) in the control group, and 3.6 (±2.4) in the intervention group, resulting in an adjusted mean difference of -0.69 (95% CI -1.04 to -0.34). The adjusted mean difference of patient's satisfaction score was 1.54 (95% CI 0.96 to 2.12); mean control group: 26.7 (±4.0); mean intervention group: 27.9 (±3.8)). Serious adverse events were comparable between both groups while 30-day mortality was 2.3% (n = 23) in the control group, and 0.8% (n = 9) in the intervention group (mean difference: -1.4% (95% CI -2.5 to -0.4)).
Interpretation: The APU process is safe and did not increase emergency department length of treatment, while patient-reported outcomes and safety were improved accompanied by an increased use of diagnostic procedures.
Funding: The study was funded by the German Innovations Funds.
Keywords: Abdominal pain; Acute care; Emergency care; Emergency department; Standardised treatment; Stepped wedge design.
© 2025 The Author(s).
Conflict of interest statement
For this study, the authors received funding from the Innovations Fund of the German Federal Joint Committee (G-BA) under the grant number 01NVF19025. In addition, the working group of AS and MM received financial support from various German public funding sources (BMBF, BMG, Innovationsfonds, NUM), Roche Diagnostics as well as the German Research Foundation. AS also received consulting fees from the Zentralinstitut für Kassenärztliche Versorgung (Zi) and from the Federal Government (Bundestag) for work unrelated to the present manuscript. In this context, she provided expert testimony for the Bundestag, likewise independent of the content of this publication. LS serves as Deputy Head of Department “Öffentliche Gesundheit und Public Health”, is a member of the extended board of the German Society for Social Medicine and Prevention (DGSMP) and acts as a spokesperson of the DGSMP working group “Migration and Health”, all on a voluntary basis. LS is also a member of the German Society of Sociology. Unrelated to this manuscript, HD gave a lecture/presentation titled “Rescue Cases” on behalf of AstraZeneca. He further holds a leadership position as a scientific director at INOB and as vice president of DGINA. AFR received a reduced participation fee for her active participation (poster presentation) at the DIVI 2024. Both AFR and BS are members of the DGINA e.V. In addition, BS is a member of the DGIM e.V. FI is employed by Charité–Universitätsmedizin Berlin. KV received financial support for attending meetings or travel from the German Innovationsfonds. Furthermore, AW is a member of der German Society of Sociology. MM has provided consultancy services to Thermofisher and Roche Diagnostics outside the scope of this manuscript. He has also delivered lectures/presentations for Diasorin, Roche Diagnostics, AstraZeneca, Sanofi, EMCREG and PeerVoice. MM is a member of the Chair of the EUSEM Research Committee and an expert panel member of the Research Institute of the Local Health Care Funds (WIdO).
Figures

References
-
- Mockel M., Searle J., Muller R., et al. Chief complaints in medical emergencies: do they relate to underlying disease and outcome? The Charité Emergency Medicine Study (CHARITEM) Eur J Emerg Med. 2013;20(2):103–108. - PubMed
-
- Fagerström A., Paajanen P., Saarelainen H., et al. Non-specific abdominal pain remains as the most common reason for acute abdomen: 26-year retrospective audit in one emergency unit. Scand J Gastroenterol. 2017;52(10):1072–1077. - PubMed
LinkOut - more resources
Full Text Sources

