Anthropomorphic tissue-mimicking phantoms for oximetry validation in multispectral optical imaging
- PMID: 40678081
- PMCID: PMC12267859
- DOI: 10.1117/1.JBO.30.7.076006
Anthropomorphic tissue-mimicking phantoms for oximetry validation in multispectral optical imaging
Abstract
Significance: Optical imaging of blood oxygenation ( ) can be achieved based on the differential absorption spectra of oxy- and deoxyhemoglobin. A key challenge in realizing clinical validation of the biomarkers is the absence of reliable reference standards, including test objects.
Aim: To enable quantitative testing of multispectral imaging methods for assessment of by introducing anthropomorphic phantoms with appropriate tissue-mimicking optical properties.
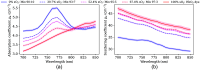
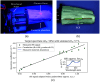
Approach: We used the stable copolymer-in-oil base material to create physical anthropomorphic structures and optimized dyes to mimic the optical absorption of blood across a wide spectral range. Using 3D-printed phantom molds generated from a magnetic resonance image of a human forearm, we molded the material into an anthropomorphic shape. Using both reflectance hyperspectral imaging (HSI) and photoacoustic tomography (PAT), we acquired images of the forearm phantoms and evaluated the performance of linear spectral unmixing (LSU).
Results: Based on 10 fabricated forearm phantoms with vessel-like structures featuring five distinct levels (between 0 and 100%), we showed that the measured absorption spectra of the material correlated well with HSI and PAT data with a Pearson correlation coefficient consistently above 0.8. Further, the application of LSU enabled a quantification of the mean absolute error in assessment with HSI and PAT.
Conclusions: Our anthropomorphic tissue-mimicking phantoms hold potential to provide a robust tool for developing, standardising, and validating optical imaging of .
Keywords: anthropomorphic phantoms; hyperspectral imaging; optical imaging; oximetry; photoacoustic imaging.
© 2025 The Authors.
Figures





Similar articles
-
Effects of skin tone on photoacoustic imaging and oximetry.J Biomed Opt. 2024 Jan;29(Suppl 1):S11506. doi: 10.1117/1.JBO.29.S1.S11506. Epub 2023 Dec 20. J Biomed Opt. 2024. PMID: 38125716 Free PMC article.
-
Validation of subpixel target detection and linear spectral unmixing techniques on short-wave infrared hyperspectral images of collagen phantoms.J Biomed Opt. 2025 Feb;30(2):023518. doi: 10.1117/1.JBO.30.2.023518. Epub 2025 Feb 25. J Biomed Opt. 2025. PMID: 40008292 Free PMC article.
-
Photoacoustic imaging of rat kidney tissue oxygenation using second near-infrared wavelengths.J Biomed Opt. 2025 Feb;30(2):026002. doi: 10.1117/1.JBO.30.2.026002. Epub 2025 Feb 18. J Biomed Opt. 2025. PMID: 39968505 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia.Cochrane Database Syst Rev. 2015 Jun 23;2015(6):CD010896. doi: 10.1002/14651858.CD010896.pub2. Cochrane Database Syst Rev. 2015. PMID: 26102272 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

