Cell Shock Absorption via Stress Relaxation Hydrogel Microspheres for Alleviating Endoplasmic Reticulum Stress in Chondrocytes
- PMID: 40678150
- PMCID: PMC12267986
- DOI: 10.34133/research.0777
Cell Shock Absorption via Stress Relaxation Hydrogel Microspheres for Alleviating Endoplasmic Reticulum Stress in Chondrocytes
Abstract
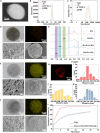
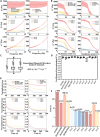
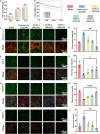
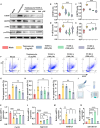
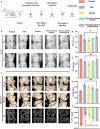
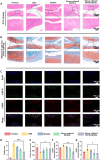
Chronic mechanical vibrations and endoplasmic reticulum (ER) stress are major contributors to osteoarthritis (OA) progression. This study proposes a novel "cellular shock absorption" approach by developing viscoelastic hydrogel microspheres with tunable stress relaxation properties. By modulating the chemical bonds in the hydrogel network through oxidation and hydrazine coupling reaction, hydrogel microspheres capable of absorbing shock and reducing mechanical stimulus-induced ER stress in chondrocytes are created. Cationic liposomes, modified with the cartilage-targeting peptide Wyrgrl and loaded with tauroursodeoxycholic acid (TUDCA), are encapsulated within these hydrogel microspheres. The microspheres not only dissipate intra-articular impact forces, reducing vibration and pressure transmission, but also provide sustained release of TUDCA, alleviating ER stress and slowing OA progression. In vitro studies showed that the stress relaxation time constant (τ) of the microspheres was tuned to 23.81 s, closely resembling the mechanical properties of the cartilage matrix. This property, combined with targeted TUDCA delivery, reduced Grp78 and CHOP expression, alleviating ER stress and inhibiting chondrocyte apoptosis. In vivo, the microspheres preserved joint cartilage structure, suppressed ER stress responses, and substantially delayed OA progression. This strategy presents a promising approach to mitigating cartilage damage and delaying OA by reducing mechanical stress and alleviating ER stress.
Copyright © 2025 Ding Zhao et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Proteomic analysis of hydrogen peroxide-treated human chondrocytes shows endoplasmic reticulum stress, cytoskeleton remodeling, and altered secretome composition.Cell Commun Signal. 2025 Jun 13;23(1):282. doi: 10.1186/s12964-025-02291-z. Cell Commun Signal. 2025. PMID: 40514670 Free PMC article.
-
Porous PLGA microspheres loaded with PTH1-34 peptide for long-term treatment of OA.J Orthop Translat. 2025 Jun 9;53:99-111. doi: 10.1016/j.jot.2025.05.003. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40538640 Free PMC article.
-
Human Infrapatellar Fat Pad Mesenchymal Stem Cell-derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model.Clin Orthop Relat Res. 2024 Jul 1;482(7):1246-1262. doi: 10.1097/CORR.0000000000003067. Epub 2024 Apr 19. Clin Orthop Relat Res. 2024. PMID: 38662932 Free PMC article.
-
Autologous chondrocyte implantation in the knee: systematic review and economic evaluation.Health Technol Assess. 2017 Feb;21(6):1-294. doi: 10.3310/hta21060. Health Technol Assess. 2017. PMID: 28244303 Free PMC article.
-
Nanodevices for deep cartilage penetration.Acta Biomater. 2022 Dec;154:23-48. doi: 10.1016/j.actbio.2022.10.007. Epub 2022 Oct 12. Acta Biomater. 2022. PMID: 36243371
References
-
- Seetharaman S, Vianay B, Roca V, Farrugia AJ, de Pascalis C, Boëda B, Dingli F, Loew D, Vassilopoulos S, Bershadsky A, et al. Microtubules tune mechanosensitive cell responses. Nat Mater. 2022;21(3):366–377. - PubMed
-
- Mao Y, Wickström SA. Mechanical state transitions in the regulation of tissue form and function. Nat Rev Mol Cell Biol. 2024;25(8):654–670. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

