This is a preprint.
P2RX7 regulates tauopathy progression via tau and mitochondria loading in extracellular vesicles
- PMID: 40678243
- PMCID: PMC12270207
- DOI: 10.21203/rs.3.rs-6771517/v1
P2RX7 regulates tauopathy progression via tau and mitochondria loading in extracellular vesicles
Abstract
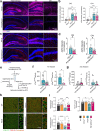
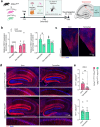
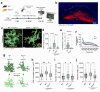
P2x purinoreceptor 7 (P2RX7), an ATP-gated ion channel, is known to play pivotal roles in the progression of Alzheimer's disease (AD), although its cell type-specific pathological mechanisms have yet to be elucidated. Here, we show that genetic deletion of P2rx7 mitigates brain atrophy, tau accumulation and cognitive impairment in PS19 tauopathy mice. Specific deletion of P2rx7 in microglia, but not astrocytes, significantly suppresses tau propagation from the entorhinal cortex to CA1 in the hippocampus, an early event in AD pathology. Single-cell (sc)-RNA sequencing of mouse brains revealed specific P2rx7 expression in microglia, inducing inflammatory changes accompanied by elevated extracellular vesicles (EVs) secretion in PS19 mice. Brain-derived EVs (BDEVs) proteome demonstrated that P2RX7 increases EV cargo loading of tau and mitochondrial molecules in BDEVs from PS19 mice, which was further validated by single-molecule super-resolution. Notably, following the injection of BDEVs isolated from PS19 mice with or without P2rx7 deficiency, the microglial transcriptome of recipient mice revealed enriched DNA-sensing and type II interferon signaling in response to BDEVs from PS19 mice, which was diminished in the group injected with P2rx7-deficient BDEVs. Thus, our results indicate that P2RX7 regulates EV-mediated tau and mitochondrial transfer and inflammatory activation in microglia with increased EV secretion, thereby contributing to tauopathy and neurodegeneration, highlighting the therapeutic potential of targeting the P2RX7-EV axis in AD.
Keywords: Alzheimer’s disease; P2X purinoceptor 7; extracellular vesicles; microglia; microtubule-associated protein tau; mitochondria; tauopathy.
Conflict of interest statement
Competing interest The authors declare no competing interests.
Figures





References
-
- Hardy J. & Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
-
- Leng F. & Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17, 157–172 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

