Inhibition of neuronal necroptosis via disruption of RIPK1-RIPK3 Interactions: The role of neural stem cell-derived exosomes in spinal cord injury recovery
- PMID: 40678260
- PMCID: PMC12268101
- DOI: 10.1016/j.bioactmat.2025.06.042
Inhibition of neuronal necroptosis via disruption of RIPK1-RIPK3 Interactions: The role of neural stem cell-derived exosomes in spinal cord injury recovery
Abstract
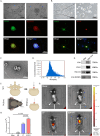
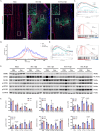
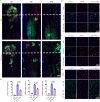
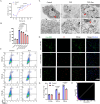
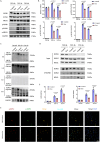
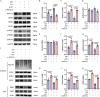
Spinal cord injury (SCI) poses a significant economic and public health burden. Exosomes derived from neural stem cells (NSC-Exos) are emerging as a promising therapeutic strategy for SCI repair, overcoming several limitations associated with both autologous and allogeneic neural stem cell therapies. This study demonstrates that NSC-Exos are efficiently internalized by the injured spinal cord after co-injection, resulting in substantial motor function recovery in murine models. Additionally, NSC-Exos effectively limit the expansion of the injury site, reduce neuronal degeneration, and attenuate neuroinflammatory responses. Notably, this is the first study to identify necroptosis as a novel therapeutic target for NSC-Exos in SCI recovery. We show that NSC-Exos inhibit neuronal necroptosis both in vivo and in vitro by disrupting the RIPK1-RIPK3 interaction, thereby preventing necrosome assembly. Furthermore, comprehensive transcriptomic analysis reveals that the ubiquitin-mediated proteolysis (UPS) pathway plays a crucial role in this process, a finding supported by experimental inhibition of ubiquitination. In conclusion, this study highlights the therapeutic potential of NSC-Exos in SCI treatment, particularly through the inhibition of necroptosis via disruption of the RIPK1-RIPK3 interaction, potentially involving UPS activation. These findings provide a foundation for future investigations into the molecular mechanisms underlying SCI recovery.
Keywords: Exosomes; Necroptosis; Necrosomes; Neural stem cells; Spinal cord Injury.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

