Analisi di costo-efficacia di nirmatrelvir/ritonavir in pazienti adulti ad alto rischio di progressione a COVID-19 severo
- PMID: 40678405
- PMCID: PMC12269775
- DOI: 10.33393/grhta.2025.3403
Analisi di costo-efficacia di nirmatrelvir/ritonavir in pazienti adulti ad alto rischio di progressione a COVID-19 severo
Abstract
Objective:: This study aimed to estimate the cost-effectiveness of nirmatrelvir/ritonavir (NMV/r) versus the standard of care (SoC) for patients with mild-to-moderate coronavirus disease 2019 (COVID-19) at high risk for progression to severe illness from the perspective of the Italian National Health Service.
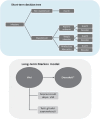
Methods:: A cost-effectiveness model was developed using a closed cohort of 1,000 infected patients. It employed a short-term decision tree (1 year) followed by a lifetime 2-state Markov model (alive and dead). The short-term decision tree captured costs and outcomes associated with the primary infection and healthcare utilization; survivors of the short-term decision tree were followed until death to calculate Italian quality-adjusted life years (QALYs), adjusted in the short-term for survivors to mechanical ventilation. Baseline hospitalization rate and NMV/r effectiveness were sourced from the Istituto Superiore di Sanità (ISS) and the randomized clinical trial Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR), respectively. Other inputs were given by previous COVID-19 studies and publicly available sources. Sensitivity analyses were conducted for all model inputs to test the robustness of the results of the model.
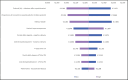
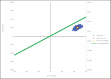
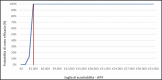
Results:: NMV/r presented an advantageous cost-effectiveness ratio of 2,237 €/QALY, compared to the SoC, in the long-term horizon (30 years). Results were most sensitive to baseline risk of hospitalization and NMV/r treatment effectiveness parameters. The probabilistic analysis indicated that NMV/r has a 100% probability of being cost-effective at a 33,004 € willingness-to-pay threshold.
Conclusions:: From the Italian National Health Service perspective, NMV/r is cost-effective compared to the standard of care for patients at high risk for severe COVID-19.
Conflict of interest statement
Conflict of interest: Carolina Moreno and Andrea Aiello declare they have no conflict of interest related to this article. They are employee of PharmaLex Italy S.p.A. Valentina Mazzotta and Andrea Antinori declare they have no conflict of interest related to this article. Andrea Antinori has served as a paid consultant to Astra Zeneca, Gilead Sciences, GSK, Janssen-Cilag, Merck, Moderna, Pfizer, and ViiV Healthcare and received research funding through the National Institute for Infectious Diseases Lazzaro Spallanzani IRCCS from Astra Zeneca, Gilead Sciences and ViiV Healthcare. Roberto Di Virgilio is a Pfizer employee and contributed to the work in accordance with ICMJE Author.
Figures
References
-
- Gorbalenya AE, Baker SC, Baric RS et al. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. - DOI - PMC - PubMed
-
- Agenzia Italiana del Farmaco (AIFA). Riclassificazione del medicinale per uso umano Paxlovid. [(Accessed November; 2024 )];Gazzetta Ufficiale n. 296 del 20/12/2023. Online
-
- EMA. Paxlovid Riassunto delle caratteristiche del prodotto. [(Accessed November; 2024 )]; Online
LinkOut - more resources
Full Text Sources

