Discovery and characterization of selective lipase-inhibiting polyheterocyclic derivatives: a combined in silico and in vitro study
- PMID: 40678412
- PMCID: PMC12266345
- DOI: 10.55730/1300-0152.2748
Discovery and characterization of selective lipase-inhibiting polyheterocyclic derivatives: a combined in silico and in vitro study
Abstract
Background/aim: Obesity has become a global health crisis with an increasing prevalence, necessitating the search for effective therapies. Schiff base derivatives, known for their broad pharmacological activities, have gained attention as potential antiobesity agents. This study aimed to investigate the lipase inhibitory potential of novel Schiff base derivatives and assess their drug-like properties through in vitro assays and in silico methods.
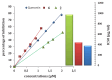
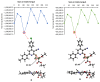
Materials and methods: The lipase inhibitory activity of synthesized Schiff base derivatives was evaluated using in vitro assays, with IC50 values determined for each compound. Additionally, in silico ADMET predictions (absorption, distribution, metabolism, excretion, and toxicity), molecular docking studies, and density functional theory (DFT) calculations were conducted to assess the pharmacokinetic properties and binding potential of the compounds to the lipase active site.
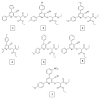
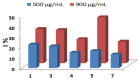
Results: The synthesized Schiff base derivatives demonstrated significant lipase inhibitory activity, with IC50 values of 995.74 ± 0.010 μM (6) and 1985.51 ± 0.041 μM (2), comparable to the reference compound quercetin (843.06 ± 0.0007 μM). In silico ADMET analyses revealed that compounds 2 and 6 possess favorable pharmacokinetic properties and exhibit drug-like characteristics. Molecular docking studies showed robust binding interactions between these compounds and the lipase active site, which were further corroborated by DFT calculations that identified reactive regions and stable conformations. Among the compounds, compound 6 exhibited the most effective inhibition and interaction profile, indicating its potential as a lipase inhibitor. These findings underscore the potential of Schiff base derivatives as promising antiobesity agents.
Conclusion: The results of our study highlight the potential of Schiff base derivatives as promising candidates for antiobesity therapy, given their significant lipase inhibitory activity and favorable in silico predictions. Further research is needed to elucidate the precise mechanisms of action and assess the efficacy of these compounds in vivo.
Keywords: ADMET; Schiff base derivatives; in silico; in vitro; lipase inhibition; molecular docking.
© TÜBİTAK.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest, financial or otherwise.
Figures


References
-
- Abad N, Al-Ostoot FH, Ashraf S, Chkirate K, Aljohani MS, et al. (2023) Synthesis, crystal structure, DFT, Hirshfeld surface analysis, energy frameworks and in-Silico drug-targeting PFKFB3 kinase of novel triazolequinoxalin derivative (TZQ) as a therapeutic strategy against cancer. Heliyon. 9(11) doi: 10.1016/j.heliyon.2023.e21312. - DOI - PMC - PubMed
-
- Aissaoui N, Hamaizia L, Brika SKM, Laamari A. Recent Updates in Eating Disorders. IntechOpen; 2023. Prevalence and determinants of obesity in children in Algeria. - DOI
-
- Amer S, El-Wakiel N, El-Ghamry H. Synthesis, spectral, antitumor, and antimicrobial studies on Cu(II) complexes of purine and triazole Schiff base derivatives. Journal of Molecular Structure. 2013;1049:326–335. doi: 10.1016/j.molstruc.2013.06.059. - DOI
LinkOut - more resources
Full Text Sources
