Plant-derived extracellular vesicles as a natural drug delivery platform for glioblastoma therapy: A dual role in preserving endothelial integrity while modulating the tumor microenvironment
- PMID: 40678580
- PMCID: PMC12269444
- DOI: 10.1016/j.ijpx.2025.100349
Plant-derived extracellular vesicles as a natural drug delivery platform for glioblastoma therapy: A dual role in preserving endothelial integrity while modulating the tumor microenvironment
Abstract
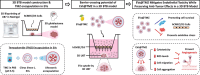
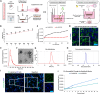
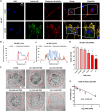
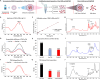
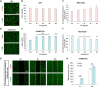
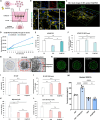
Glioblastoma (GBM) is the most aggressive primary brain tumor, with limited treatment options due to the restrictive blood-brain barrier (BBB) and the heterogeneity of the blood-tumor barrier (BTB). Temozolomide (TMZ), the standard chemotherapy, suffers from poor BBB permeability, rapid degradation, and systemic toxicity. Plant-derived extracellular vesicles (PDEVs) have emerged as promising natural nanocarriers, offering biocompatibility, stability, and the ability to cross biological barriers. This study investigates the use of extracellular vesicles from Citrus limon L. (LDEs) to encapsulate and deliver TMZ (EVs@TMZ) for GBM treatment. LDEs were isolated, characterized, and loaded with TMZ via ultrasonication. Encapsulation efficiency, stability, and physicochemical properties were assessed using UV-Vis and FTIR spectroscopy. A 3D BTB model was developed using bioprinted U87 glioblastoma cells in Matrigel, co-cultured with hCMEC/D3 endothelial cells to replicate the tumor microenvironment. Barrier integrity was evaluated through TEER and FITC-dextran assays. Uptake, cytotoxicity, and tumor invasion were assessed in this model, along with oxidative stress and VEGF-A secretion. LDEs effectively encapsulated TMZ, enhancing drug stability under physiological conditions. EVs@TMZ crossed the endothelial barrier while preserving barrier integrity and reducing TMZ-induced ROS production. In the 3D glioblastoma model, EVs@TMZ showed strong cytotoxic effects on tumor cells while minimizing endothelial toxicity and oxidative stress. Moreover, VEGF-A secretion was suppressed, disrupting pro-tumorigenic pathways. These findings highlight Citrus-derived EVs as biocompatible, efficient carriers for TMZ delivery, offering a promising approach to overcome current challenges in GBM therapy and supporting further development of PDEVs for brain tumor treatment.
Keywords: 3D bioprinting; Blood-tumor barrier; Drug delivery; Glioblastoma; Plant-derived extracellular vesicles; VEGF-A.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

