The Impact of Overexpression of the Mouse Ortholog of CACNA1C on Behavior and Cortical Dynamics
- PMID: 40678692
- PMCID: PMC12268564
- DOI: 10.1016/j.bpsgos.2025.100537
The Impact of Overexpression of the Mouse Ortholog of CACNA1C on Behavior and Cortical Dynamics
Abstract
Background: Mental disorders are common in the United States. According to the National Institute of Mental Health more than 23% of the adult population in the United States live with some form of mental illness. Genome-wide association studies have implicated CACNA1C, which encodes the L-type voltage-gated calcium channel CaV1.2, and it has been suggested that the expression levels of CACNA1C may be associated with mental illness. To this end, we have generated a novel mouse line that conditionally overexpresses the mouse ortholog Cacna1c.
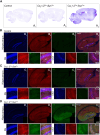
Methods: Transgenic mice (CaV1.2Tg+ mice) were characterized for expression and distribution of CaV1.2. The CaV1.2Tg+ mice were compared with control littermates using assays that examined cognitive and affective behaviors. Cortical network dynamics were assessed using in vivo multiphoton calcium imaging.
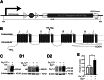
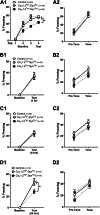
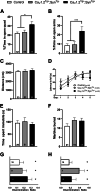
Results: Compared with their control littermates, CaV1.2Tg+ mice exhibited a ∼1-fold increase in CaV1.2 expression. Behavioral characterization of the CaV1.2Tg+ mice revealed a complex phenotype in which they exhibited deficits in the consolidation of fearful memories and an increase in anxiolytic-like behavior. The CaV1.2Tg+ mice also appeared to have altered cortical dynamics in which the network was more dense but less synchronized.
Conclusions: We have successfully generated mice that overexpress the mouse ortholog of a gene that has been implicated in several psychiatric diseases. Our initial characterization suggests that these mice have alterations in behavior and neural function that have been linked to mental illness. It is anticipated that future studies will reveal additional neurobehavioral alterations whose mechanisms will be studied.
Keywords: Bipolar disorder; Cortical subnetworks; L-type calcium channel CaV1.2; Psychiatric risk variant; Schizophrenia.
Plain language summary
More than 23% of the adult population in the United States live with some form of mental illness. Several genes have been identified, which appear more frequently in people diagnosed with a psychiatric condition, that have small alterations of unknown significance. There is some evidence suggesting that expression levels of one of these genes, CACNA1C, may be altered in psychiatric patients. To address this question, using genetic engineering, we have generated mice that overexpress the mouse version of CACNA1C. Our initial characterization suggests that these mice have alterations in behavior and neural function that have been linked to mental illness.
© 2025 The Authors.
Figures
References
-
- Berger S.M., Bartsch D. The role of L-type voltage-gated calcium channels CaV1.2 and CaV1.3 in normal and pathological brain function. Cell Tissue Res. 2014;357:463–476. - PubMed
-
- Nanou E., Catterall W.A. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. 2018;98:466–481. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

