Impact of conflicting information on the use of antirheumatic drugs in pregnancy and breastfeeding: perspectives of healthcare providers from the global PRAISE survey
- PMID: 40678818
- PMCID: PMC12267951
- DOI: 10.1177/1759720X251350087
Impact of conflicting information on the use of antirheumatic drugs in pregnancy and breastfeeding: perspectives of healthcare providers from the global PRAISE survey
Abstract
Background: Treating rheumatic musculoskeletal diseases (RMDs) during pregnancy and breastfeeding presents significant complexities, mainly due to inconsistencies between the clinical guidance documents and the reference safety information, including the summary of product characteristics (SmPC) and the patient information leaflets (PIL).
Objectives: To assess healthcare professionals' (HCPs) prescribing behaviors, comfort levels, and challenges when advising patients, focusing on discrepancies between clinical guidance documents and SmPC/PIL.
Design: Online survey entitled PRAISE (Perception of healthcare providers Regarding Antirheumatics in pregnancy and breastfeeding: advice, Information and patient perSpEctives) and disseminated through HCPs groups and social media.
Methods: A cross-sectional survey was conducted among 414 HCPs globally. Respondents were divided into prescribers (n = 336) and non-prescribers (n = 78) based on their self-reported role in prescribing antirheumatic medications to pregnant or breastfeeding patients with RMDs. The survey covered demographics, clinical experience, confidence in prescribing, use of clinical guidelines, and experiences managing conflicting information between guidelines and SmPC/PIL.
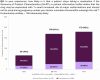
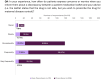
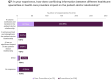
Results: Prescribers were more likely than non-prescribers to feel comfortable discussing medication safety during pregnancy. Most prescribers found clinical guidance documents useful, with 48% rating them as "very useful" and 38% as "extremely useful." In case of conflicting information between clinical guidance documents and SmPC/PIL, 58% of HCPs reported that it caused confusion and tension in patient-doctor relationships, and almost 20% of them are "likely" or "very likely" to discontinue ongoing treatment. Clear communication and shared decision-making were the most common strategies used to address patient concerns.
Conclusion: HCPs often face significant challenges when advising patients with RMDs on the use of medications during pregnancy and breastfeeding. Conflicting information between clinical guidance documents and SmPC/PIL can disrupt patient-doctor relationship and lead to treatment discontinuation, with potential consequences on maternal disease control. Improved alignment between clinical guidance documents and the SmPC/PIL could enhance patient care and prevent confusion among HCPs and patients.
Keywords: antirheumatic medications; breastfeeding; lactation; patient information leaflet; pregnancy; summary of product characteristics.
Plain language summary
When the patient leaflet and the clinical guidelines say different things on the use of antirheumatic medications during pregnancy and breastfeeding: global health care providers say that conflicting information can cause confusion and tension in the patient-clinician relationship Background: When pregnant or breastfeeding, women with rheumatic musculoskeletal diseases (RMDs) face challenges in managing their condition due to conflicting information about medication safety. Healthcare providers (HCPs) often struggle with discrepancies between clinical guidelines and the safety information provided with medications, such as the Summary of Product Characteristics and Patient Information Leaflets. This discrepancy can lead to difficulties in advising patients about safe medication use during pregnancy and breastfeeding. HCPs might feel uncertain or uncomfortable discussing medication safety, which can affect the patient-doctor relationship and even lead to stopping necessary treatments. The PRAISE study: To understand these challenges better, the PRAISE study conducted an online survey among 414 HCPs worldwide. We aimed to assess how HCPs prescribe medications, their comfort levels in advising patients, and the challenges they face due to conflicting information. Our key findings include: Confidence in prescribing: HCPs who prescribe medications were more comfortable discussing medication safety during pregnancy than those who do not prescribe.Usefulness of guidelines: Most prescribers found clinical guidelines very useful in managing patient care.Conflicting information: Over half of the HCPs reported feeling confused or tense when dealing with conflicting information between guidelines and medication safety documents. This confusion can lead to discontinuing treatments, which might negatively impact disease control.Communication strategies: HCPs often use clear communication and shared decision-making to address patient concerns. In conclusion, PRAISE highlights the need for better alignment between clinical guidelines and medication safety information to improve patient care and reduce confusion. This could help ensure that pregnant and breastfeeding women with RMDs receive the best possible.
© The Author(s), 2025.
Conflict of interest statement
K.S.: Speaker and/or consultancy fees from Eli Lilly, Thermo-Fisher, UCB. C.G.: No conflict of interest. I.P.: Research funding and/or honoraria from Amgen, AstraZeneca, Aurinia, BMS, Eli Lilly, Gilead, GSK, Janssen, Novartis, Otsuka, and Roche. N.A.-L.: No conflict of interest. S.A.: No conflict of interest. A.A.: No conflict of interest. G.K.B.: Honoraria and consultancy fees from Abbvie, AstraZeneca, GSK, Novartis, Otsuka, Pfizer. I.B.: No conflict of interest. A.B.: Speaker and/or consultancy fees from Astra Zeneca, Boehringer Ingelheim, Ewo Pharma, Sandoz, Stada, UCB. S.C.: No conflict of interest. R.C.: Speaker and/or consultancy fees from Alpine, AstraZeneca, BMS, Celgene, Eli Lilly, GSK, Janssen, Pfizer, Roche, Rubió, UCB. N.C.-C.: Consultancy fees from BMS; research grants from Roche and UCB paid to the institution. R.D.: Unrestricted research grants from Dutch Arthritis Association, Galapagos, UCB, ZonMw. Speaking fees from Abbvie, AstraZeneca, Eli Lilly, Genzyme, Novartis, Roche, UCB. O.E.: No conflict of interest. J.F.: Speaker and consultancy fees from UCB. J.E.F.: No conflict of interest. R.F.-S.: Speaker and/or consultancy fees from: GSK, Novartis, Pfizer, UCB. F.F.: Speaker and/or consultancy fees from GSK, Novartis, Otsuka, Roche, UCB. I.Giles: Research funding, speaker and consultancy fees from UCB. B.G.: Speaker fees from GSK. C.G.S.: No conflict of interest. I.Gunnarsson: Research collaboration with Annexon, speaker fees from Otsuka. L.G.: No conflict of interest. H.S.K.: No conflict of interest. L.L.: Research funding from Novartis was paid to the institution. J.L.: No conflict of interest. Y.M.: Speaker fees from Eli Lilly, Pfizer. A.M.: Speaker and/or consultancy fees from Abbvie, Biogen, BMS, Janssen, Novartis, Pfizer, UCB, Viatris. L.M.: Speaker fees from Janssen, UCB. M.M.: Speaker and consultancy fees from UCB. C.N.-P.: Speaker and consultancy fees from UCB. L.F.P.-G.: Speaker and/or consultancy fees from AlfaSigma, Galapagos, Pfizer. L.R.: No conflict of interest. A.L.R.: No conflict of interest. A.R.: No conflict of interest. M.S.: No conflict of interest. S.S.: No conflict of interest. A.S.: Speaker fees from AbbVie, Amgen, Eli Lilly, Galapagos/AlfaSigma, Janssen, MSD, Pfizer, Roche, Takeda, UCB. A.S. is the PI of the RABBIT register, which is funded through an institution with unrestricted grants from AbbVie, Amgen, BMS, Celltrion, Eli Lilly, Fresenius Kabi, Alfasigma, Hexal, MSD, Pfizer, Samsung Biogen, Sanofi, Biocon, UCB. E.S.: Research grant from Merck paid to the institution. M.G.T.: Research funding and/or honoraria from Abbvie, Boehringer Ingelheim, DEMO, Eli Lilly, Faran, GSK, UCB. A.T.: Speaker and consultancy fees from Galapagos, UCB. A.T.: Speaker fees from AstraZeneca, Boehringer Ingelheim, Otsuka, Vipor Pharmaceuticals. J.V.: No conflict of interest. A.V.: No conflict of interest. M.W.: No conflict of interest. N.Z.-S.: Speaker fees from GSK. L.A.: Speaker and/or consultancy fees from AlfaSigma, GSK, Janssen, Novartis, Pfizer, UCB, Werfen Group.
Figures




Similar articles
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
-
Perceptions and experiences of the prevention, detection, and management of postpartum haemorrhage: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2023 Nov 27;11(11):CD013795. doi: 10.1002/14651858.CD013795.pub2. Cochrane Database Syst Rev. 2023. PMID: 38009552 Free PMC article.
-
Parents' and informal caregivers' views and experiences of communication about routine childhood vaccination: a synthesis of qualitative evidence.Cochrane Database Syst Rev. 2017 Feb 7;2(2):CD011787. doi: 10.1002/14651858.CD011787.pub2. Cochrane Database Syst Rev. 2017. PMID: 28169420 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Giles I, Yee CS, Gordon C. Stratifying management of rheumatic disease for pregnancy and breastfeeding. Nat Rev Rheumatol 2019; 15: 391–402. - PubMed
-
- Giles I, Thorne I, Schmidt NS, et al. The time of equipoise on the use of biological DMARDs in for inflammatory arthritis during pregnancy is finally over: a reappraisal of evidence to optimise pregnancy management. Lancet Rheumatol 2024; 6: e546–e559. - PubMed
-
- Andreoli L, Crisafulli F, Tincani A. Pregnancy and reproductive aspects of systemic lupus erythematosus. Curr Opin Rheumatol 2017; 29: 473–479. - PubMed
LinkOut - more resources
Full Text Sources

