Improving SARS-CoV-2 variants monitoring in the absence of genomic surveillance capabilities: a serological study in Bolivian blood donors in October 2021 and June 2022
- PMID: 40679142
- PMCID: PMC12274064
- DOI: 10.7554/eLife.94475
Improving SARS-CoV-2 variants monitoring in the absence of genomic surveillance capabilities: a serological study in Bolivian blood donors in October 2021 and June 2022
Abstract
Background: Unlike genomic data, serological data have not been previously leveraged to evaluate the SARS-CoV-2 variants circulation. In Bolivia, sustained genomic surveillance capacities were lacking, especially at the beginning of the pandemic.
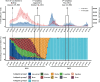
Methods: In 2021 and 2022 we estimated the prevalence of anti-SARS-CoV-2 antibodies in Bolivian blood donors and explored the feasibility of using virus serum neutralization data for variants thought to have circulated to map their circulation across all departments over a year-long follow-up period. Anti-S1 and anti-NCP SARS-CoV-2 IgGs were studied, along with virus neutralization tests for ancestral-D614G, Gamma, Delta, and Omicron BA.1 lineages of SARS-CoV-2.
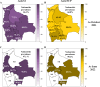
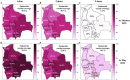
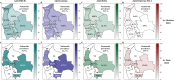
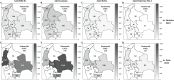
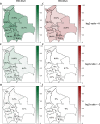
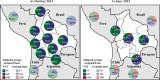
Results: Between 2021 and 2022, the overall prevalence of anti-S1 and anti-NCP antibodies increased, reaching values over 90%, demonstrating that a large proportion of the Bolivian population was no longer naïve to the virus. Viral neutralization data, analyzed through multiple approaches, revealed the spread of the Gamma variant up to 2021, particularly impacting northern departments. In 2022, Gamma continued to circulate in southernmost departments of the country, and the emergence of Omicron BA.1 was detected. These trends align with publicly available genomic data from neighboring countries.
Conclusions: Our serological analyses successfully identified both new antigenic groups, such as Omicron BA.1, and individual variants related to previously circulating groups, such as Delta. The study contributes insights into overall population immunity to SARS-CoV-2 and variant-specific immunity levels across different regions of Bolivia. It also emphasizes the potency of seroprevalence studies in informing public health decisions and underscores their value in capturing the initial phases of emerging epidemics when variant diversity is limited, facilitating timely genomic surveillance setup.
Funding: This study was supported by the French National Research Institute for Sustainable Development (IRD), the project EMERGEN-PRI #22275 of the ANRS I MIE (INSERM), and the European Union's Horizon 2020 research and innovation program (European Virus Archive Global, grant agreement No. 871029). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Keywords: SARS-CoV-2; epidemiology; global health; human; infectious disease; microbiology; public health; surveillance; variant circulation.
© 2024, Inchauste et al.
Conflict of interest statement
LI, EN, LB, YL, SL, MH, KM, PM, MR, JP, JG, YL, EA, PG, Xd, SP No competing interests declared
Figures


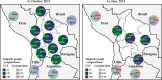
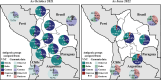
Update of
References
-
- Álvarez-Antonio C, Meza-Sánchez G, Calampa C, Casanova W, Carey C, Alava F, Rodríguez-Ferrucci H, Quispe AM. Seroprevalence of anti-SARS-CoV-2 antibodies in Iquitos, Peru in July and August, 2020: a population-based study. The Lancet. Global Health. 2021;9:e925–e931. doi: 10.1016/S2214-109X(21)00173-X. - DOI - PMC - PubMed
-
- Bolivia MdSyDd Inicia El Proceso de Vacunación Al Personal de Salud Que Lucha en Primera Línea Contra la Pandemia. 2021a. [April 17, 2023]. https://www.minsalud.gob.bo/5230-inicia-proceso-vacunacion
-
- Bolivia MdSyDd Reporte de vacunacion contra la COVID-19 al 27 de octubre 2021. 2021b. [May 2, 2023]. https://www.minsalud.gob.bo/6163-covid-19-hasta-hoy-la-inmunizacion-alca...
-
- Bolivia MdSyDd Reporte de Vacunacion Contra la COVID-19 al 30 de Mayo de 2022. 2022. [May 2, 2023]. https://www.minsalud.gob.bo/6729-covid-19-salud-informa-que-esta-jornada...
-
- Buss LF, Prete CA, Jr, Abrahim CMM, Mendrone A, Jr, Salomon T, de Almeida-Neto C, França RFO, Belotti MC, Carvalho M, Costa AG, Crispim MAE, Ferreira SC, Fraiji NA, Gurzenda S, Whittaker C, Kamaura LT, Takecian PL, da Silva Peixoto P, Oikawa MK, Nishiya AS, Rocha V, Salles NA, de Souza Santos AA, da Silva MA, Custer B, Parag KV, Barral-Netto M, Kraemer MUG, Pereira RHM, Pybus OG, Busch MP, Castro MC, Dye C, Nascimento VH, Faria NR, Sabino EC. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371:288–292. doi: 10.1126/science.abe9728. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

