SEAMM: A Simulation Environment for Atomistic and Molecular Modeling
- PMID: 40679150
- PMCID: PMC12319917
- DOI: 10.1021/acs.jpca.5c03164
SEAMM: A Simulation Environment for Atomistic and Molecular Modeling
Abstract
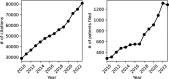
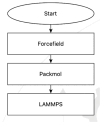
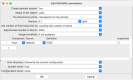
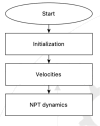
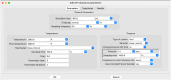
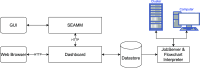
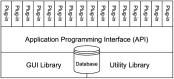
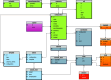
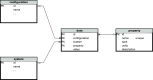
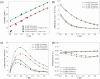
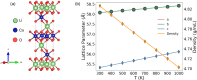
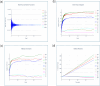
The simulation environment for atomistic and molecular modeling (SEAMM) is an open-source software package written in Python that provides a graphical interface for setting up, executing, and analyzing molecular and materials simulations. The graphical interface reduces the entry barrier for the use of new simulation tools, facilitating the interoperability of a wide range of simulation tools available to solve complex scientific and engineering problems in computational molecular science. Workflows are represented graphically by user-friendly flowcharts, which are shareable and reproducible. When a flowchart is executed within the SEAMM environment, all results, as well as metadata describing the workflow and codes used, are saved in a datastore that can be viewed using a browser-based dashboard, which allows collaborators to view the results and use the flowcharts to extend the results. SEAMM is a powerful productivity and collaboration tool that enables interoperability between simulation codes and ensures reproducibility and transparency in scientific research. We illustrate the flexibility and productivity of SEAMM with three examples: a simple molecular dynamics calculation to provide an overview; exploring the rearrangement of methylisocyanide to acetonitrile using a wide range of quantum codes and force fields; and using SEAMM for industrial research on battery materials with simulations of the diffusivity and ionic conductivity of electrolytes and the density, thermal expansion, and thermal conductivity of cathode materials as a function of lithiation.
Figures




References
-
- Talirz L., Ghiringhelli L. M., Smit B.. Trends in atomistic simulation software usage [Article v1.0] LiveComs. 2021;3(1):1483. doi: 10.33011/livecoms.3.1.1483. - DOI
-
- Talirz, L. ; Aprá, E. ; Corsetti, F. ; Moussa, J. E. ; Poncé, S. . ltalirz/atomistic-software: v2024.3.29. 2024.
-
- Dumaz M., Boucher R., Marques M. A., Romero A. H.. Authorship and citation cultural nature in density functional theory from solid state computational packages. Scientometrics. 2021;126:6681–6695. doi: 10.1007/s11192-021-04057-z. - DOI
-
- Bornmann L., Haunschild R., Mutz R.. Growth rates of modern science: a latent piecewise growth curve approach to model publication numbers from established and new literature databases. Humanit. Soc. Sci. Commun. 2021;8:224. doi: 10.1057/s41599-021-00903-w. - DOI
LinkOut - more resources
Full Text Sources

