Spontaneous Crimping of Gelatin Methacryloyl Nanofibrils Induced by Limited Hydration
- PMID: 40679322
- PMCID: PMC12344688
- DOI: 10.1021/acsbiomaterials.5c00828
Spontaneous Crimping of Gelatin Methacryloyl Nanofibrils Induced by Limited Hydration
Abstract
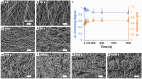
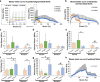
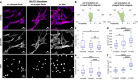
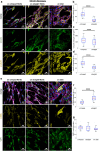
The crimped appearance of native collagen fibrils in youthful tissues serves as a mechanical buffer and phenotype determinant for resident cells. In vitro platforms emulating these native crimped networks facilitate the study of cell-matrix dynamics in various pathophysiological contexts. However, creating fibrillar networks with sizes and crimping matching native tissues using collagen-derived substrates remains challenging. We present an innovative approach to produce spontaneous, tunable crimping of electrospun, aligned gelatin methacryloyl nanofibrils using limited hydration. The diameter of the synthesized fibrils approximated that of native fibrils. Beyond individual fibril crimping, the network exhibited large-scale, periodic crimping with wavelengths matching native collagen networks. Tensile stress tests revealed that crimping reduced network stiffness but enhanced stretchability, consistent with native tissues. Additionally, crimping promoted cell translocation into the network. Fibroblasts cultured on crimped fibrils showed smaller cell areas, higher vinculin and α-tubulin expression, and lower α-smooth muscle actin levels compared to those on straight fibrils. This novel method not only replicates the native fibril characteristics using collagen-derived materials, but also offers a valuable tool for advancing our understanding of cell-matrix interactions, with significant implications for tissue engineering and regenerative medicine.
Keywords: 3D cell culture; Collagen derivatives; GelMA; fibril crimping; tissue scaffold.
Figures






References
-
- Gathercole L., Keller A.. Crimp morphology in the fibre-forming collagens. Matrix. 1991;11(3):214–234. doi: 10.1016/S0934-8832(11)80161-7. - DOI - PubMed
- Gruber H. E., Hanley E. N. Jr.. Observations on morphologic changes in the aging and degenerating human disc: secondary collagen alterations. BMC Musculoskeletal Disord. 2002;3(1):9. doi: 10.1186/1471-2474-3-9. - DOI - PMC - PubMed
-
- Hansen K. A., Weiss J. A., Barton J. K.. Recruitment of tendon crimp with applied tensile strain. J. Biomech. Eng. 2002;124(1):72–77. doi: 10.1115/1.1427698. - DOI - PubMed
- Rigby B. J., Hirai N., Spikes J. D., Eyring H.. The mechanical properties of rat tail tendon. J. Gen. Physiol. 1959;43(2):265–283. doi: 10.1085/jgp.43.2.265. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
