Treatment burden in patients with paroxysmal nocturnal hemoglobinuria: an in-depth interview survey
- PMID: 40679586
- PMCID: PMC12334466
- DOI: 10.1007/s00277-025-06486-9
Treatment burden in patients with paroxysmal nocturnal hemoglobinuria: an in-depth interview survey
Abstract
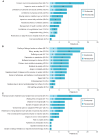
Paroxysmal nocturnal hemoglobinuria (PNH) is a lifelong, clonal hematologic disease posing life-threatening risks if untreated. The prognosis for PNH has improved with the advent of C5 inhibitors, which are now the standard of care where available. As treatment options continue to expand, healthcare providers can better address both PNH management and the impact of treatment on patients' daily lives. To investigate the burden of disease and key factors taken into consideration when patients with PNH choose their preferred treatment, we conducted an in-depth patient interview survey. Of survey participants (N = 30), 70.0% were receiving intravenous C5 inhibitors. Notably, 56.7% of all patients reported needing ≥ 1 hospital visit per month, and 46.7% required a day off from work or school for visits (33.3% among those receiving intravenous C5 inhibitors). A frequently reported burden of PNH treatment was financial concern, including the cost of treatment, hospital visits, and the negative impact on income. Additionally, burden related to waiting times and distance from the hospital and the overall time spent on outpatient PNH care were identified with similar results for patients receiving intravenous C5 inhibitors. These results suggest that the time and effort to get to treatment centers and the time required to receive treatment for PNH were critical unmet needs in PNH care. Our study indicates that persistent burdens associated with current PNH care should be taken into account alongside therapeutic effectiveness when making treatment decisions. Further analysis with a larger sample size is required to confirm these findings.
Keywords: Burden of disease; Burden of treatment; Impact on daily life; PNH; Qualitative patient interview.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Institutional review board statement: The study was registered under UMIN-CTR as UMIN000051639, on July 19, 2023. The study protocol was approved by Non-Profit Organization MINS IRB, on June 22 (Registration number: 230216). Informed consent: Informed consent was obtained from all subjects involved in the study. Competing interests: Y.U. reports consultancy for Alexion, Asahi Kasei, Chugai, Janssen, Novartis, Ono, and Sanofi; speakers’ bureau fees from Alexion, Incyte Biosciences Japan, Janssen, Sanofi, and Sobi; advisor for Alexion, Janssen, and Novartis; research funding from Chugai; honoraria from Chugai, Kaken, and Nippon Shinyaku. N.O. reports honoraria from Alexion, Novartis, Sobi, Chugai/F. Hoffmann-La Roche Ltd, and Kyowa Kirin; research funding from Alexion. S.U. reports speakers’ bureau fees from Chugai, Sobi Japan, Asahi Kasei, Alexion and Novartis. K.H. has no disclosures to declare. M.S. reports honoraria from Chugai, Alexion, Asahi Kasei, and Novartis. T.M. and Y.F. are employees at CRECON Medical Assessment Inc. N.H. and M.Y. are employees at Chugai. Y.O. reports research funding from Mochida and FUJIFILM; honoraria from AstraZeneca, Chugai, SymBio, Pfizer Japan, Kyowa Kirin, Novartis, Novo Nordisk, Meiji Seika, Otsuka, CSL Behring, Sanofi, Daiichi Sankyo, Nippon Kayaku, Bristol Myers Squibb, Bayer Yakuhin, Takeda, Zeria, Eisai, AbbVie, Alexion, Nipro, and PharmaEssentia Japan; other support (clinical trials) from Novartis, Mochida, Janssen, Bristol Myers Squibb, MSD, Shionogi, Meiji Seika, AbbVie, Labcorp Development Japan, Chugai, Kyowa Kirin, Astellas, Shionogi Healthcare Clinical, Otsuka, IQVIA Services Japan, Parexel International, Incyte Biosciences Japan, Sumitomo, ICON Japan, Ono, and Daiichi Sankyo. K.U. has received research funding from Agios, Astellas, AbbVie, Alexion, Aperis, Asahi Kasei, Bristol Myers Squibb, Celgene, Chugai, Daiichi Sankyo, Eisai, Gilead, Incyte, Kyowa Kirin, MSD, Nippon Shinyaku, Novartis, Ohara, Ono, Otsuka, PharmaEssentia, Pfizer, Sanofi, Takeda, and Yakult; speakers’ bureau fees from AbbVie, Alexion, Amgen, Astellas, Asahi Kasei Pharma, AstraZeneca, Bristol Myers Squibb, Chugai, Eisai, Incyte, Kyowa Kirin, Meiji Seika Pharma, Novartis, Sanofi, Nippon Shinyaku, Ono, Otsuka, Pfizer, Sando, PharmaEssentia, Sanofi, Takeda, and Janssen; consulting bureau fees from Alnylam Japan, Ohara, and Chugai. T.I. reports consultancy for Chugai, Nippon Shinyaku, Asahi Kasei, and Alexion; honoraria from Asahi Kasei, Alexion, Sanofi, and Nippon; research funding from Asahi Kasei; scholarship donations from Asahi Kasei, Nippon Shinyaku, Astellas, AbbVie, Otsuka, Takeda, Novartis, and Janssen. T.K. reports honoraria from Alexion and Chugai. N.H. reports honoraria from Chugai, Janssen, Bristol Myers Squibb, Takeda, and Novartis; research funding from Otsuka, Chugai, and Shionogi. Y.K. reports scholarship donations from Chugai, Kyowa Kirin, Shionogi, Asahi Kasei, Otsuka, and Takeda. A.G. reports honoraria from Novartis, Alexion, Eisai, Ono, Taiho, Takeda, Nippon Shinyaku, Chugai, Otsuka, Sumitomo Dainippon, Daiichi Sankyo, Nihon, Kyowa Kirin, Janssen, Pfizer Japan, Sanofi, Asahi Kasei, and PharmaEssentia Japan; consulting fees from PharmaEssentia Japan, Chugai, Alexion, and Asahi Kasei; advisor for PharmaEssentia Japan, Chugai, Alexion, and Asahi Kasei; research funding from Asahi Kasei, Ono, Taiho, Chugai, and Otsuka. J.N. reports honoraria from Chugai, F. Hoffmann-La Roche Ltd, Alexion, Novartis, Sobi, and Sanofi.
Figures

References
-
- Roth A, Maciejewski J, Nishimura JI, Jain D, Weitz JI (2018) Screening and diagnostic clinical algorithm for paroxysmal nocturnal hemoglobinuria: expert consensus. Eur J Haematol 101:3–11 - PubMed
-
- Japan intractable diseases information center. In https://www.nanbyou.or.jp/entry/5354. Accessed 26 June 2025
-
- Risitano AM, Marotta S, Ricci P, Marano L, Frieri C, Cacace F, Sica M, Kulasekararaj A, Calado RT, Scheinberg P, Notaro R, Peffault de Latour R (2019) Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol 10:1157 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

