An early feasibility study for neurological devices: The ARCTRAN study
- PMID: 40679690
- PMCID: PMC12488755
- DOI: 10.1007/s10072-025-08367-5
An early feasibility study for neurological devices: The ARCTRAN study
Abstract
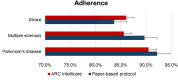
Introduction: An Early Feasibility Study (EFS) is an exploratory clinical investigation of a device to optimize its design through iterative feedback loops during early clinical experience. The ARCTRAN study is an EFS aimed at analyzing efficacy, safety and adherence to the home-rehabilitation device-based (ARC Intellicare) program compared to a paper-based exercise protocol in patients with Parkinson's disease (PD), Multiple Sclerosis (MS) and stroke.
Materials and methods: At baseline (T0), patients of each group were randomly divided into two arms, with a 1:1 ratio: an 'interventional' group received ARC Intellicare and a 'control' group received a paper-based rehabilitation protocol, both consisting of three 60-minute sessions/week for 8 weeks. Each patient was evaluated by means of clinical scales, gait analysis and stabilometry at baseline (T0) and after the rehabilitation (T2).
Results: All patients (n = 90, 30 per group) showed a significant improvement in clinical scales between T0 and T2. Stroke patients showed a bigger gait improvement in the ARC Intellicare arm, measured both by clinical scales (Tinetti gait p = 0.01) and gait analysis parameters. In PD group, only gait analysis parameters recorded a significant improvement in the active arm.
Discussion: We demonstrate the safety of ARC Intellicare for home-based rehabilitation in patients with chronic neurological conditions, with high adherence levels. Moreover, in stroke group, it produced more significant improvements of gait compared to paper-based protocol. In the global scenario of the EFS projects promoted by the Italian Ministry of Health, this study is a starting point to conduct this type of studies in Italy.
Keywords: Early feasibility study; Multiple sclerosis; Parkinson’s disease; Stroke; Telerehabilitation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: Authors declare no competing interests. Conflict of interest: The authors have no relevant financial or non-financial interests to disclose. Ethical approval: This study was approved by the local Ethics Committee. Informed consent: Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- (2013) US Food and Drug Administration, Center for Biologics Evaluation and Research. Investigational device exemptions (IDEs) for early feasibility medical device clinical studies, including certain first in human (FIH) studies: guidance for industry and food and drug administration staff. Available from: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/...
-
- (2017) Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745
-
- (2021) Italian Ministry of Health, Directorate General for Medical Devices. Notifica di indagini cliniche svolte con dispositivi medici non recanti la marcatura CE (pre market). Available from: http://www.salute.gov.it/portale/ministro/p4_8_0.jsp?lingua=italiano26la...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical