Efficacy and Tolerability of Vortioxetine Versus Selective Serotonin Reuptake Inhibitors for Late-Life Depression: A Post-hoc Analysis of the VESPA Study
- PMID: 40679716
- PMCID: PMC12313815
- DOI: 10.1007/s40266-025-01231-3
Efficacy and Tolerability of Vortioxetine Versus Selective Serotonin Reuptake Inhibitors for Late-Life Depression: A Post-hoc Analysis of the VESPA Study
Abstract
Background and objectives: Usual treatment approaches for late-life depression primarily involve selective serotonin reuptake inhibitors (SSRIs). Recently, the potential role of vortioxetine has garnered attention. This study aimed to investigate whether vortioxetine is superior to SSRIs in terms of efficacy and tolerability in older people with moderate-to-severe depression.
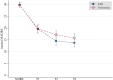
Methods: The Vortioxetine in the Elderly versus SSRIs: a Pragmatic Assessment (VESPA) study was an assessor-blinded, randomized, parallel-group, superiority trial, comparing flexible doses of vortioxetine versus SSRIs in older adults with depression. This is a post-hoc analysis that excluded participants with milder symptoms of depression. The primary outcome was the change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores. Secondary outcomes included clinical response (MADRS total score reduction of ≥ 50%), remission (a MADRS score < 10), and discontinuation rates. Clinical measures were conducted at baseline and at 1-month, 3-month, and 6-month (endpoint) visits.
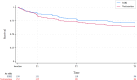
Results: In total, 302 individuals (mean age: 73.4 ± 5.9 years; 68.9% females), comprising 152 randomized to vortioxetine and 150 to SSRIs (sertraline N = 92; paroxetine N = 19; escitalopram N = 19; citalopram N = 16; fluoxetine N = 3; fluvoxamine N = 1), were included in this post-hoc analysis. No significant differences in MADRS improvement between vortioxetine and SSRIs were observed at any follow-up visits and 6-month endpoint (-11.8 ± 10.6 versus -14.0 ± 11.6; p = 0.12). This was further confirmed by a subgroup analysis excluding drug discontinuers (-16.8 ± 9.0 versus -17.6 ± 10.3; p = 0.51). In addition, people treated with vortioxetine did not exhibit better rates of response (44.1 versus 53.0%; p = 0.11), remission (25.7 versus 34.7%; p = 0.09), and discontinuation (38.0 versus 30.2%; p = 0.17), including discontinuation owing to either side effects or inefficacy, compared with those treated with SSRIs.
Conclusions: Vortioxetine was not superior to SSRIs in terms of efficacy and tolerability in older adults with moderate-to-severe depression. Additional trials, possibly based on fixed doses of vortioxetine, are needed.
Registration: Clinicaltrials.gov: NCT03779789, registered on 12 Dec 2018; EudraCT number: 2018-001444-66.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: The study is funded by AIFA within the 2016 call for Independent Research on Drugs (Bando AIFA per la Ricerca Indipendente 2016); code: 2016–0234923. The study Sponsor had no role in study design; in the collection, management, analysis, and interpretation of data; in writing the report; and in the decision to submit the report for publication. The study Sponsor had no ultimate authority over any of the listed activities. Conflict of interest: F.B. has received direct or indirect consultant fees from Angelini, Rovi and IQVIA Solutions, and honoraria for editorial activities from Elsevier and AVES. S.C. has received consultant fees from Lundbeck, Otsuka, Angelini and Johnson & Johnson. G.M. has received research grants, consulting fees and honoraria from Angelini, Doc Generici, Janssen-Cilag, Lundbeck, Neuraxpharm, Otsuka, Pfizer, Servier, Rovi and Recordati. MSS has received consultant fees from Lundbeck and Angelini. Other authors reported no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Ethics approval: The study was first approved for the coordinating centre (University of Verona) by the Ethics Committee for Clinical Research of Verona and Rovigo (protocol 61211 of the 19 September 2018; protocol version 1.5 of the 09 June 2018) and thereafter by the local ethics committee of each recruiting centre. The study was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Consent to participate: All participants signed a written informed consent form. Consent for publication: Not applicable. Availability of data and material: The dataset will be made available upon motivated request. Code availability: Not applicable. Author contributions: All authors have read and approved the final submitted manuscript and agree to be accountable for the work. F.B.: Conceptualization; Methodology; Validation; Formal Analysis; Data Curation; Writing - Original Draft. D.C.: Investigation; Writing – Original Draft; Visualization. I.R.: Investigation; Writing – Original Draft; Visualization. T.C.: Investigation; Data curation; Writing – Review and Editing. C.Cr.: Methodology; Formal Analysis; Writing – Original Draft. C.G.: Investigation; Resources; Writing – Review and Editing. A.A.: Investigation; Writing – Review and Editing. C.Ca.: Investigation; Writing – Review and Editing. S.Ca.: Investigation; Writing – Review and Editing. S.Ch.: Investigation; Writing – Review and Editing. M.C.: Investigation; Writing – Review and Editing. A.D.: Investigation; Writing – Review and Editing. I.E.: Investigation; Writing – Review and Editing. L.G.: Investigation; Writing – Review and Editing. M.I.: Investigation; Writing – Review and Editing. S.M.: Investigation; Writing – Review and Editing. G.M.: Investigation; Writing – Review and Editing. M.R.: Investigation; Writing – Review and Editing. A.R.: Investigation; Writing – Review and Editing. R.R.: Investigation; Writing – Review and Editing. V.R.: Investigation; Writing – Review and Editing. C.S.G.: Investigation; Writing – Review and Editing. M.S.S.: Investigation; Writing – Review and Editing. L.T.: Investigation; Writing – Review and Editing. G.O.: Validation; Investigation; Resources; Writing – Review and Editing; Project administration; Funding acquisition. G.C.: Methodology; Writing – Original Draft; Supervision.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

