Prenatal stress increases corticosterone levels in offspring by impairing placental glucocorticoid barrier function
- PMID: 40679963
- PMCID: PMC12273964
- DOI: 10.1371/journal.pone.0313705
Prenatal stress increases corticosterone levels in offspring by impairing placental glucocorticoid barrier function
Abstract
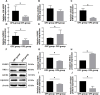
This study aimed to investigate the association between prenatal stress (PS) and corticosterone levels, and its influence on DNA methylation of genes related to the placental glucocorticoid (GC) barrier, including 11β-HSD2, ABCB1 (P-gp), NR3C1, and FKBP5. The PS model was established through chronic unpredictable mild stress (CUMS). DNA methylation of GC-related genes was analyzed by reduced representation bisulfite sequencing (RRBS), and the results were confirmed using MethylTarget™ sequencing. The mRNA and protein expression levels of these genes were detected through qRT-PCR and Western blotting, respectively. Plasma corticosterone levels were elevated in pregnant female rats exposed to PS conditions and their offspring. Compared to the offspring of the prenatal control (OPC) group, the offspring of the prenatal stress (OPS) group exhibited down-regulation in both mRNA and protein expression of DNA methyltransferases (DNMT 3A and DNMT 3B), while up-regulation was observed in the expression of DNMT1. RRBS analyses identified ABCB1 and FKBP5 as hypermethylated genes, including a total of 43 differentially methylated sites (DMS) and 2 differentially methylated regions (DMR). MethylTarget™ sequencing further confirmed 15 differentially methylated CpG sites in these genes. This study provides preliminary evidence that PS disrupts the placental GC barrier through abnormal gene expression caused by hypermethylation of GC-related genes, resulting in elevated corticosterone levels in offspring and affecting their growth and development.
Copyright: © 2025 Liu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



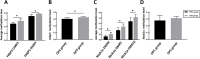
References
-
- Guan S-Z, Fu Y-J, Zhao F, Liu H-Y, Chen X-H, Qi F-Q, et al. The mechanism of enriched environment repairing the learning and memory impairment in offspring of prenatal stress by regulating the expression of activity-regulated cytoskeletal-associated and insulin-like growth factor-2 in hippocampus. Environ Health Prev Med. 2021;26(1):8. doi: 10.1186/s12199-020-00929-7 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

