Promising therapeutic efficacy of nitazoxanide-loaded zinc oxide nano-formula against intestinal and muscular phases of experimental trichinellosis
- PMID: 40679976
- PMCID: PMC12274003
- DOI: 10.1371/journal.pntd.0013239
Promising therapeutic efficacy of nitazoxanide-loaded zinc oxide nano-formula against intestinal and muscular phases of experimental trichinellosis
Abstract
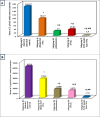
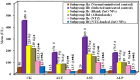
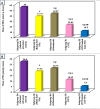
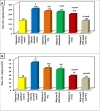
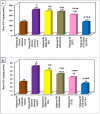
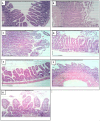
Trichinellosis is a ubiquitous parasitic infection caused by a zoonotic nematode known as Trichinella spiralis (T. spiralis). It starts with the adult worm in the intestinal phase and ends up with the larva reaching the muscles. The disease generally manifests with acute gastroenteritis; however, it may regrettably lead to life-threatening myositis, myocarditis and seizures. The commercially existing chemotherapeutic regimens have numerous drawbacks including severe adverse effects, high resistance rate, poor bioavailability and low efficiency towards the muscular stage. Consequently, the current study targeted the evaluation of nitazoxanide-loaded zinc oxide nanoparticles (NTZ-loaded ZnO NPs) used for the first time in the treatment of both the intestinal and muscular phases of trichinellosis in mice. Swiss Albino mice were orally infected by 250 T. spiralis larvae. The experimental animals were treated with the gold standard albendazole, NTZ, ZnO NPs as well as NTZ-loaded ZnO NPs. Parasitological, biochemical (creatine kinase, alanine transaminase, aspartate transaminase, alkaline phosphatase, malondialdehyde and nitric oxide), immunological (interleukins 2 and 4) and histopathological assessments were conducted. The parasitological results denoted that the mice treated with NTZ-loaded ZnO NPs revealed the uppermost significant drug efficacy (>97%) in both the intestinal and muscular phases indicating efficacious tissue penetration. Additionally, this group revealed the most profound amelioration of the biochemical and immunological markers as well as restoration of the histopathological picture. Conclusively, the present work implied a bird's eye view on the promising effectiveness of NTZ-loaded ZnO NPs in the treatment of murine trichinellosis relying on the anti-parasitic safe nature of the formulation.
Copyright: © 2025 Hagras et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

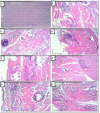
References
-
- Bruschi F. Helminth Infections and their Impact on Global Public Health. Springer Vienna. 2014. doi: 10.1007/978-3-7091-1782-8 - DOI
-
- Salata RA, King CH. Parasitic Infections of the Central Nervous System. Neurology and General Medicine. Elsevier. 2008;921–46. doi: 10.1016/b978-044306707-5.50052-3 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

