Transcranial photobiomodulation therapy with 808 nm light changes expression of genes and proteins associated with neuroprotection, neuroinflammation, oxidative stress, and Alzheimer's disease: Whole RNA sequencing of mouse cortex and hippocampus
- PMID: 40679987
- PMCID: PMC12273915
- DOI: 10.1371/journal.pone.0326881
Transcranial photobiomodulation therapy with 808 nm light changes expression of genes and proteins associated with neuroprotection, neuroinflammation, oxidative stress, and Alzheimer's disease: Whole RNA sequencing of mouse cortex and hippocampus
Abstract
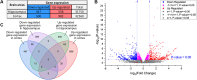
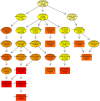
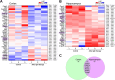
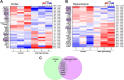
Light therapy, using red and near-infrared (NIR) irradiation, is currently applied for the treatment of various neurodegenerative diseases, such as Alzheimer's disease (AD). Transcranial photobiomodulation therapy (tPBMT) can alleviate neurodegeneration, neuronal loss, and β-amyloid peptide plaque burden. Alternatively, potential early inhibition of oxidative stress, neuroinflammation, apoptosis, and amyloidogenic cellular pathways may constrain pathological changes with aging. In this research, we conduct an 808-nm tPBMT with a 30-day course of daily 1-hour sessions for mice and assess its influence on molecular mechanisms related to the potential onset of neurodegeneration. To comprehensively identify molecular mechanisms of tPBMT on the brain cells, the next-generation whole RNA sequencing of over 30,000 mRNA of the cortex and hippocampus of BALB/c mice is performed. After tPBMT, transcriptional alterations are found in 1,005 genes in the hippocampus and 1,482 genes in the cortex. Pathway-gene enrichment network analysis identifies genes associated with about 20 pathways of neurodegeneration, and a disease-gene network is constructed. Particularly, tPBMT alters the transcription and expression of the essential genes associated with oxidative stress (NF-κBIα, JUN, JUND, and PKC genes), inflammation (DOCK4/6, IL-1RAPL1, and TNFαIP6), and apoptosis (CASP3, TNFαIP6, AKT3, CDKN1A, CYP51, RASA2, and RESTAT). Additionally, 808-nm light modulates the main risk genes for AD (BACE1, BACE2, PSEN2, APH1B, GATA2, YY2, RELA, STAT3, JUN, JUND, ARNTL, CREB3L1, CELF2, E2F4, ELK3, and CEBPD), involved in APP processing supporting AD development. Moreover, the APP concentration is reduced after tPBMT. Hence, PBMT may help inhibit the development of different neurodegeneration types and maintain normal brain conditions.
Copyright: © 2025 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
None of the authors has a conflict of interest. All the data are available on request to authors.
Figures





References
-
- Rheaume YL, Manning BC, Harper DG, Volicer L. Effect of light therapy upon disturbed behaviors in Alzheimer patients. Am J Alzheimer’s Dis. 1998;13(6):291–5. doi: 10.1177/153331759801300604 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

