Image-based inference of tumor cell trajectories enables large-scale cancer progression analysis
- PMID: 40680117
- PMCID: PMC12273761
- DOI: 10.1126/sciadv.adv9466
Image-based inference of tumor cell trajectories enables large-scale cancer progression analysis
Abstract
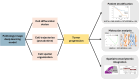
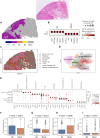
Current approaches to estimating cell trajectories, tumor progression dynamics, and cell population diversity of tumor microenvironment often depend on single-cell RNA sequencing, which is costly and resource intensive. To address this limitation, we developed an artificial intelligence (AI) model that leverages cell morphology features and histological spatial organization to classify tumor cell differentiation status, infer cell dynamic trajectories, and quantify tumor progression from hematoxylin and eosin (H&E)-stained whole-slide images. In three independent lung adenocarcinoma cohorts, our AI-based model accurately predicted cell differential status and provided quantifiable measures of tumor progression that were prognostic of patient survival. Spatial transcriptomic integrative analyses revealed cell components and gene signatures enriched in different cell differentiation statuses. Bulk transcriptomic analyses revealed that fast-progressing tumors exhibit up-regulated cell cycle pathways, while slow-progressing tumors retain characteristics of normal lung epithelium. This cost-effective method enables large-scale analysis of tumor progression dynamics using routinely collected pathology slides and provides insights into intratumor heterogeneity.
Figures




References
-
- Siegel R. L., Giaquinto A. N., Jemal A., Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024). - PubMed
-
- Dagogo-Jack I., Shaw A. T., Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018). - PubMed
-
- Saelens W., Cannoodt R., Todorov H., Saeys Y., A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

