Nicotinamide boosts oocyte quantity and quality by promoting N4-acetylation modification in lupus mice
- PMID: 40680131
- PMCID: PMC12273787
- DOI: 10.1126/sciadv.adu0955
Nicotinamide boosts oocyte quantity and quality by promoting N4-acetylation modification in lupus mice
Abstract
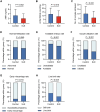
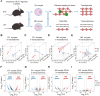
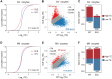
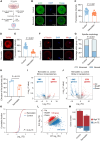
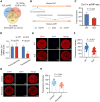
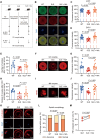
Patients with systemic lupus erythematosus (SLE) often have decreased fertility. Gene translation is crucial to oocyte meiosis and development. However, it remains unclear how SLE affects this process. Here, we used single-cell transcriptome and translatome sequencing to uncover a notable disruption in protein translation in oocytes from SLE mice, associated with the N4-acetylcytidine (ac4C) modification. Inhibition of ac4C levels in vitro substantially reduced oocyte translation efficiency. Notably, through trace-cell ac4C-RNA immunoprecipitation (acRIP) sequencing, we mapped the ac4C landscape in SLE mouse oocytes and found that deficient ac4C modification substantially impaired the translation of Zygote arrest 1. Furthermore, we demonstrated that nicotinamide treatment notably and safely improved the quantity and quality of oocytes in SLE mice by enhancing ac4C modification levels. Our findings highlight the essential role of N- acetyltransferase 10 (NAT10)-mediated ac4C modification in abnormal oocyte development in SLE and suggest that nicotinamide holds promise for improving fertility in patients with SLE.
Figures



References
-
- Andreoli L., Bertsias G. K., Agmon-Levin N., Brown S., Cervera R., Costedoat-Chalumeau N., Doria A., Fischer-Betz R., Forger F., Moraes-Fontes M. F., Khamashta M., King J., Lojacono A., Marchiori F., Meroni P. L., Mosca M., Motta M., Ostensen M., Pamfil C., Raio L., Schneider M., Svenungsson E., Tektonidou M., Yavuz S., Boumpas D., Tincani A., EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 76, 476–485 (2017). - PMC - PubMed
-
- Tarter L., Bermas B. L., Expert perspective on a clinical challenge: Lupus and pregnancy. Arthritis Rheumatol. 76, 321–331 (2024). - PubMed
-
- Han Y. F., Yan Y., Wang H. Y., Chu M. Y., Sun K., Feng Z. W., Feng H., Effect of systemic lupus erythematosus on the ovarian reserve: A systematic review and meta-analysis. Joint Bone Spine 91, 105728 (2024). - PubMed
-
- Giambalvo S., Garaffoni C., Silvagni E., Furini F., Rizzo R., Govoni M., Bortoluzzi A., Factors associated with fertility abnormalities in women with systemic lupus erythematosus: A systematic review and meta-analysis. Autoimmun. Rev. 21, 103038 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

