Day 3 Oxford criteria predict steroid non-response for acute severe ulcerative colitis in the post biologic era
- PMID: 40680157
- PMCID: PMC12492213
- DOI: 10.1093/ecco-jcc/jjaf131
Day 3 Oxford criteria predict steroid non-response for acute severe ulcerative colitis in the post biologic era
Abstract
Background and aims: Outcomes of patients admitted with acute severe ulcerative colitis (ASUC) in the post biologic era are under explored, as well as the ability of scoring indices to predict early steroid non-response.
Methods: This retrospective cohort study included adults hospitalized with ASUC (2010-2022) at London Health Sciences Centre, Canada. Steroid response, need for rescue therapy, colectomy during index hospitalization, and colectomy and hospitalization at 3- and 12-months following discharge was assessed. Logistic regression identified predictors of steroid non-response, defined as need for rescue therapy or colectomy during hospitalization.
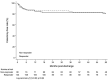
Results: Of 261 adults hospitalized with ASUC (male: 51.7%, mean age: 40.6 years), 71.2% had extensive colitis. After intravenous corticosteroid therapy during index admission, 55.7% (n = 147) had a response, 37.9% (n = 99) received rescue therapy (infliximab: 98, tofacitinib: 1, and cyclosporine: 0), and 8% (21/261) underwent colectomy. Additionally, 11.6% (28/240) of patients discharged from hospital underwent colectomy within the first 12 months (8.3% at 3-months and 3.3% between 3 and 12 months). There was no difference between steroid responders and non-responders for colectomy (11% vs 12.6%) or hospitalization (33.5% vs 32.6%) at 12 months. The overall cumulative probabilities of colectomy for the entire cohort at 1 year, 3 years, and 5 years were 13.5%, 16.1%, and 17.4%, respectively. On multivariate analysis, Day 3 Oxford criteria was the only factor found to be statistically significant in predicting steroid non-response (odds ratio 4.70, 95%CI [1.06-20.80]).
Conclusions: Day 3 Oxford criteria was an independent predictor of steroid non-response. The risk of colectomy remains substantial after discharge despite low in-hospital colectomy rates following an episode of ASUC. Initial steroid response did not affect long-term colectomy rate at 12 months.
Keywords: colectomy; cyclosporine; infliximab; ulcerative colitis.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
S.K.V.: Has received a consulting fee from Alimentiv Inc. L.A.: None. T.A.D.S: None. V.S.: None. L.G.: None. T.P.: Has received advisory board/consultant fees from Bristol Myers Squibb, Celltrion, Eli Lilly, Organon, and Pfizer. He currently serves as an investigator on clinical research studies for Abivax, Alimentiv, Applied Molecular Transport, Bristol Myers Squibb, Eli Lilly, Prometheus/Merck, and Takeda. M.B.: Has received advisory board fees from AbbVie, Astra Zeneca, Gilead, Janssen, Novo Nordisk, Lupin, Pfizer, and Takeda. J.G.: Has received speakers fees from AbbVie, Celltrion, Ferring, Janssen, Organon, and Takeda. B.Y.: None. M.S.: Has received consultant fees from Medtronic; research grants and speaker fees from Pendopharm; educational grant from Cook Medical. V.J.: Has received consulting/advisory board fees from AbbVie, Alimentiv, Anaptyis Bio, Arena Pharmaceuticals, Asahi Kasei Pharma, Asieris, Astra Zeneca, Bristol Myers Squibb, Celltrion, Eli Lilly, Endpoint Health, Ensho, Enthera, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genentech, Gilead, Innomar, JAMP, Janssen, Merck, Metacrine, MRM Health, Mylan, Pandion, Pendopharm, Pfizer, Prometheus Biosciences, Protagonist, Reistone Biopharma, Roche, Roivant, Sandoz, Second Genome, Sorriso, Spyre, Synedgen, Takeda, Teva, Ventyx, and Vividion; speaker’s fees from AbbVie, Bristol Myers Squibb, Eli Lilly, Ferring, Fresenius Kabi, Janssen, Pfizer, Shire, Takeda, and Tillotts.
Figures
References
-
- Rivière P, Li Wai Suen C, Chaparro M, et al. Acute severe ulcerative colitis management: unanswered questions and latest insights. Lancet Gastroenterol Hepatol. 2024;9:251-262. - PubMed
-
- Turner D, Walsh CM, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103-110. - PubMed
-
- Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4:431-437. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical