Clinical Characterization and Long-Term Outcome in Children and Adults With Anti-AMPA Receptor Encephalitis
- PMID: 40680249
- PMCID: PMC12275903
- DOI: 10.1212/NXI.0000000000200453
Clinical Characterization and Long-Term Outcome in Children and Adults With Anti-AMPA Receptor Encephalitis
Abstract
Background and objectives: Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (anti-AMPAR) encephalitis manifests as limbic encephalitis in adults and is often associated with cancer. Although some reports suggest that it may occur in children, the clinical features in this population, as well as the prognostic factors and long-term outcomes in children and adults, are unknown.
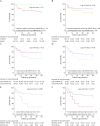
Methods: We performed a retrospective, international collaborative study of patients with anti-AMPAR encephalitis. Clinical information was reviewed, together with data from published pediatric patients. Clinical features of children and adults were compared with nonparametric tests. Survival rates (Kaplan-Meier curves) were compared using log-rank tests. Prognostic factors of poor outcome (modified Rankin Scale score >2) were identified using logistic regression models.
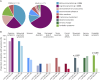
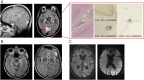
Results: A total of 115 patients were included, of whom 84 (71 adults, 13 children) had only AMPAR antibodies and 31 (27 adults, 4 children) had additional concurrent neural antibodies. Among patients with AMPAR antibodies alone, tumors were identified in 37 adults (56%) and none of the children (p < 0.0001). Children were more likely than adults to have behavioral/psychiatric symptoms (5/13, 39%, vs 8/71, 11%, p = 0.026) at onset, cerebellar dysfunction (6/13, 46%, vs 7/68, 10% p = 0.005) or movement disorders (5/13, 39%, vs 8/67, 12%, p = 0.032) during the disease course, and extratemporal brain MRI lesions (4/9, 44%, vs 5/44, 11%, p = 0.035). Among 34 patients with prolonged follow-up (>24 months), long-term neurocognitive sequelae were reported in 23 (68%), all adults. Failure to respond to first-line immunotherapy at multivariable analysis predicted a poor outcome (OR 8.0, 95% CI 1.1-59.2, p = 0.043). Among the 31 patients with concurrent neural autoantibodies, 22 (79%) had a tumor; those with high-risk antibodies had lower survival rates (p = 0.008).
Discussion: Children and adults with anti-AMPAR encephalitis show distinct clinical-radiologic features. At long-term follow-up, 68% of patients, all adults, have neurologic sequelae, with failure to respond to first-line immunotherapy being associated with worse outcomes.
Conflict of interest statement
C. Milano receives research support from Spanish National Health Institute Carlos III and co-funded by the European Union (Rio-Hortega grant CM24/00055). C. Papi receives research support from Spanish National Health Institute Carlos III (FIS grant PI23/01366) and 2023 European Academy of Neurology Research Training Fellowship. L. Marmolejo receives research support from Spanish National Health Institute Carlos III (predoctoral research grant FI24/00021). M. Spatola receives research support from La Caixa Foundation (Junior Leader) and Spanish National Health Institute Carlos III (ISCIII) and co-funded by the European Union (FIS grant PI23/01366). J. Dalmau receives research support from CaixaResearch Health 2022 (HR22-00221), Spanish National Health Institute Carlos III (ISCIII) and co-funded by the European Union (FIS grant PI23/00858), Pla estratègic de recerca i innovació en salut (PERIS)/Generalitat de Catalunya (SLT028/23/000071), Fundació Clínic per a la Recerca Biomèdica (FCRB) Programa Multidisciplinar de Recerca, Generalitat de Catalunya Department of Health (SLT028/23/000071), E.J. Safra Foundation. He receives royalties from Euroimmun for the use of NMDA as an antibody test. He received a licensing fee from Euroimmun for the use of GABAB receptor, GABAA receptor, DPPX and IgLON5 as autoantibody tests; he has received a research grant from Sage Therapeutics. J. Honnorat, M. Benaiteau, and B. Joubert are supported by a public grant overseen by the Agence Nationale de la Recherche (ANR; French research agency) as part of the Investissements d'Avenir program (ANR-18-RHUS-0012), also performed within the framework of the Laboratory of Excellence (LABEX) CORTEX of the Universitè Claude Bernard Lyon 1 (program Investissements d'Avenir, ANR-11- LABX-0042, operated by the ANR) and supported by the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases (RITA). S.I. Hooshmand has received compensation for serving on the Scientific Advisory Boards and/or for speaking engagements for EMD Serono, Genentech, TG Therapeutics, and Sanofi-Genzyme. A.L. Piquet reports research grants from the University of Colorado, Rocky Mountain Multiple Sclerosis Center, and the Foundation for Sarcoidosis; consulting fees from Genentech/Roche, UCB Pharma, EMD Serono, Kyverna and Alexion; and honorarium from MedLink and publication royalties from Springer as co-editor of a medical textbook. M.J. Titulaer and J. Kerstens are supported by the Erasmus Medical Center Trust Foundation. M.J. Titulaer, R.F. Neuteboom, and J. Kerstens are part of the European Reference Network RITA. Dr Peter Brøgger Christensen is deceased; to the best of our knowledge, he had no relevant disclosures. All the other authors report no disclosures relevant to the manuscript. Go to
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
