c-Myc promotes metabolic reprogramming in pulmonary hypertension via the stimulation of glutaminolysis and the reductive tricarboxylic acid cycle
- PMID: 40680382
- PMCID: PMC12284298
- DOI: 10.1016/j.redox.2025.103765
c-Myc promotes metabolic reprogramming in pulmonary hypertension via the stimulation of glutaminolysis and the reductive tricarboxylic acid cycle
Abstract
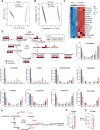
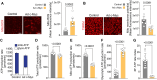
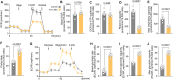
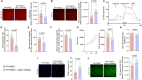
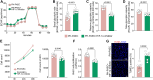
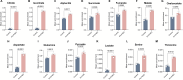
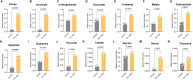
Endothelial cell (EC) dysfunction is key in initiating and progressing pulmonary hypertension (PH). EC dysfunction in PH leads to hyperproliferation and vascular remodeling of the pulmonary blood vessels. Increased glutaminolysis and altered cellular metabolism are pivotal in hyperproliferative cancer cells. However, whether a similar enhancement in glutamine metabolism is involved in the EC hyperproliferation and if this contributes to vascular remodeling during PH development is unresolved and was the focus of our study. Metabolic flux analysis showed elevated glutaminolysis and enhanced metabolic flux through the reductive tricarboxylic acid (TCA) cycle in pulmonary arterial ECs isolated from an ovine experimental model of PH (PH-PAECs). PH-PAECs also exhibited increased c-Myc protein levels, a master regulator of glutaminolysis. Therefore, we assessed the effect of increased c-Myc expression on metabolic reprogramming, glutaminolysis, and proliferation in control PAECs. Results from a comprehensive snapshot metabolomics investigation and metabolic flux analysis confirmed the reprogramming of mitochondrial metabolism, enhanced glutamine metabolism, and increased glycolysis in c-Myc overexpressing PAECs. Additionally, c-Myc overexpression impacted the ATP production rate, disrupted mitochondrial respiration, increased reactive oxygen species production, induced cell proliferation, and suppressed apoptosis. Functionally, these metabolic changes suppressed nitric oxide (NO) production. We also demonstrate that a small-molecule c-Myc inhibitor, 10058-F4, attenuates glutaminolysis, suppresses the reverse TCA cycle and glycolysis, and reverses the hyperproliferative phenotype, thereby restoring NO levels in PH-PAECs. We also demonstrate that directly targeting HIF-1α reverses the hyper-proliferative, anti-apoptotic phenotype in PH-PAECs. Thus, targeting c-Myc signaling and suppressing glutaminolysis or glycolysis could be a novel therapy for PH.
Keywords: Endothelial cells; Glutaminolysis; Glycolysis; Metabolomics; Proliferation; Pulmonary hypertension.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures








Similar articles
-
Single-Cell and Spatial Transcriptomics Identified Fatty Acid-Binding Proteins Controlling Endothelial Glycolytic and Arterial Programming in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2025 Jul;45(7):1145-1165. doi: 10.1161/ATVBAHA.124.321173. Epub 2025 May 22. Arterioscler Thromb Vasc Biol. 2025. PMID: 40401371 Free PMC article.
-
Glycolysis, Glutaminolysis, and Fatty Acid Synthesis Are Required for Distinct Stages of Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication.J Virol. 2017 Apr 28;91(10):e02237-16. doi: 10.1128/JVI.02237-16. Print 2017 May 15. J Virol. 2017. PMID: 28275189 Free PMC article.
-
Super-Enhancer-Driven HCG20 Promotes Pulmonary Hypertension Through U2AF2 Splicing.Circ Res. 2025 Jul 18;137(3):e19-e39. doi: 10.1161/CIRCRESAHA.125.326133. Epub 2025 May 28. Circ Res. 2025. PMID: 40433695
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
-
Phosphodiesterase type 5 inhibitor plus endothelin receptor antagonist compared to either alone for group 1 pulmonary arterial hypertension.Cochrane Database Syst Rev. 2025 Aug 4;8(8):CD015824. doi: 10.1002/14651858.CD015824.pub2. Cochrane Database Syst Rev. 2025. PMID: 40757552
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

