Barley resistance and susceptibility to fungal cell entry involve the interplay of ROP signaling with phosphatidylinositol-monophosphates
- PMID: 40680717
- PMCID: PMC12274078
- DOI: 10.1111/tpj.70356
Barley resistance and susceptibility to fungal cell entry involve the interplay of ROP signaling with phosphatidylinositol-monophosphates
Abstract
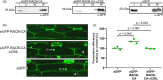
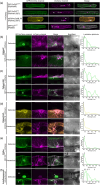
Rho-of-plant small GTPases (ROPs) are regulators of plant polar growth and of plant-pathogen interactions. The barley ROP, RACB, is involved in susceptibility toward infection by the barley powdery mildew fungus Blumeria hordei (Bh) but little is known about the cellular pathways that connect RACB signaling to disease susceptibility. Here we identify novel RACB interaction partners of plant or fungal origin by untargeted co-immunoprecipitation of constitutively active (CA) RACB tagged by green fluorescent protein from Bh-infected barley epidermal layers and subsequent analysis by liquid chromatography-coupled mass spectrometry. Three of the immunoprecipitated proteins, a plant phosphoinositide phosphatase, a plant phosphoinositide phospholipase, and a putative Bh-effector protein, are involved in the barley-Bh-pathosystem and support disease resistance or susceptibility, respectively. RACB and its plant interactors bind to overlapping anionic phospholipid species in vitro, and in the case of RACB, this lipid interaction is mediated by its carboxy-terminal polybasic region (PBR). Fluorescent markers for anionic phospholipids show altered subcellular distribution in barley cells during Bh attack and under expression of a RACB-binding fungal effector. Phosphatidylinositol 4-phosphate, phosphatidylinositol 3,5-bisphosphate, and phosphatidylserine show a distinct enrichment at the haustorial neck region, suggesting a connection to subcellular targeting of RACB at this site. The interplay of ROPs with anionic phospholipids and phospholipid-metabolizing enzymes may thus enable the subcellular enrichment of components pivotal for success or failure of fungal penetration.
Keywords: ROP GTPAse; effector; haustorium; phosphatidylinositol‐monophosphate; phosphoinositide phosphatase; phosphoinositide phospholipase; polarity; polybasic domain; susceptibility.
© 2025 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Akamatsu, A. , Wong, H.L. , Fujiwara, M. , Okuda, J. , Nishide, K. , Uno, K. et al. (2013) An OsCEBiP/OsCERK1‐OsRacGEF1‐OsRac1 module is an essential early component of chitin‐induced rice immunity. Cell Host & Microbe, 13, 465–476. - PubMed
-
- Choi, S. , Prokchorchik, M. , Lee, H. , Gupta, R. , Lee, Y. , Chung, E.H. et al. (2021) Direct acetylation of a conserved threonine of RIN4 by the bacterial effector HopZ5 or AvrBsT activates RPM1‐dependent immunity in Arabidopsis. Molecular Plant, 14, 1951–1960. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

