Synaptic enrichment of pSer129 alpha-synuclein correlates with dopaminergic denervation in early-stage Parkinson's disease
- PMID: 40681493
- PMCID: PMC12274443
- DOI: 10.1038/s41467-025-61052-1
Synaptic enrichment of pSer129 alpha-synuclein correlates with dopaminergic denervation in early-stage Parkinson's disease
Abstract
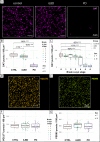
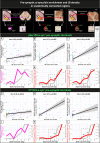
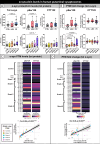
In Parkinson's disease (PD), α-synuclein aggregation in striatal synapses is hypothesised to trigger a cascade of events leading to synaptic loss and cortical Lewy body (LB) pathology. Using multiplex immunofluorescence and confocal microscopy on 69 brains spanning Braak stages 0-6-including controls, incidental LB disease (iLBD), and PD-we show that phosphorylated (pSer129) α-synuclein is enriched in putaminal dopaminergic synapses already in early disease stages, and associates with dopaminergic terminal loss. C-terminally truncated (CTT122) α-synuclein shows a similar trend in later stages. Enrichment of pSer129 and CTT122 α-synuclein in cortical glutamatergic synapses in the putamen occurs prior to LB appearance in cortical regions, supporting the theory of α-synuclein retrograde transport from synapse to cell body. Using AlphaLISA, we confirm that isolated PD putaminal synaptosomes contain higher pSer129 α-synuclein protein levels compared to controls. These findings suggest that synaptic enrichment of pSer129 α-synuclein occurs in early PD, possibly contributing to dopaminergic denervation and cortical LB pathology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors I.F., M.L.M., A.B.W., D.T.V., J.J.P.B, W.A.B., B.L.G., I.L.C., A.J.J., H.W.B., L.E.J., and W.D.J.B. declare that they have no competing interests. D.M. and M.B. are full-time employees of Roche/F. Hoffmann-La Roche Ltd, and they may additionally hold Roche stock/stock options.
Figures




References
-
- Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet397, 2284–2303 (2021). - PubMed
-
- Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord.30, 1591–1601 (2015). - PubMed
-
- Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Prim.3, 1–21 (2017). - PubMed
-
- Dickson, D. W. et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol.115, 437–444 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

