Efficacy of modified enfortumab vedotin ineligible criteria (mEVITA) in advanced urothelial carcinoma
- PMID: 40681533
- PMCID: PMC12274629
- DOI: 10.1038/s41598-025-09806-1
Efficacy of modified enfortumab vedotin ineligible criteria (mEVITA) in advanced urothelial carcinoma
Abstract
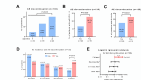
Enfortumab Vedotin Ineligible criTeriA (EVITA) were proposed for the selection of patients to receive enfortumab vedotin (EV) and pembrolizumab treatment. However, the usefulness of these criteria has not been fully verified. We retrospectively analyzed data from 301 patients with unresectable or metastatic urothelial carcinoma who underwent first-line chemotherapy and 135 patients with EV monotherapy in real-world practice. We evaluated the numbers of patients fulfilling the modified EVITA (mEVITA; excluding ocular abnormalities) and the relationship of the mEVITA to the safety and efficacy in patients with EV monotherapy. Of the 301 patients who received first-line chemotherapy, 4.3% (n = 13) met the mEVITA criteria. The primary factor contributing to mEVITA ≥ 2 was renal dysfunction. Of the 135 patients who underwent subsequent EV therapy, the number of the mEVITA had no influence on the frequency of all-grade and grade ≥ 3 adverse events. However, the mEVITA ≥ 2 was significantly associated with temporary EV interruption. Oncological outcomes were not associated with the number of the mEVITA. In conclusion, 4.3% and 14.8% of patients met the mEVITA criteria at the time of first-line and subsequent EV therapy, respectively. Having mEVITA ≥ 2 may be associated with temporary interruption of EV therapy.
Keywords: EVITA; Efficacy; Enfortumab Vedotin; Safety; Urothelial carcinoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Shingo Hatakeyama received honoraria from Janssen Pharmaceutical K.K., Astellas Pharma Inc., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Bayer AG, Pfizer Inc., Bristol-Myers Squibb, Merck Biopharma Co., Ltd., Kaneka Corporation, and Nipro Corporation. The other authors have no conflicts of interest to declare. Consent for publication: All authors approved for the publication. Disclosure of conflicts of interest: Shingo Hatakeyama received honoraria from Janssen Pharmaceutical K.K., Astellas Pharma Inc., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., Bayer AG, Pfizer Inc., Bristol-Myers Squibb, Merck Biopharma Co., Ltd., Kaneka Corporation, and Nipro Corporation. The other authors have no conflicts of interest to declare. Consent to participate: Written consent was not obtained in exchange for the public disclosure of study information (opt-out approach) by an Institutional Review Board by the ethics committee of the Hirosaki University School of Medicine (approval nos. 2019-099-2 and 2021-158-2, opt-out approach).
Figures



References
-
- Powles, T. et al. ESMO clinical practice guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol Jun. 35 (6), 485–490. 10.1016/j.annonc.2024.03.001 (2024). - PubMed
-
- Matsumoto, H. et al. Clinical practice guidelines for bladder Cancer 2019 edition by the Japanese urological association: revision working position paper. Int J. Urol May. 27 (5), 362–368. 10.1111/iju.14210 (2020). - PubMed
-
- Ito, K., Kita, Y. & Kobayashi, T. Real-world outcomes of pembrolizumab for platinum-refractory advanced urothelial carcinoma: efficacy, safety, and evidence for trial-unfit patients. Int J. Urol Sep.30 (9), 696–703. 10.1111/iju.15101 (2023). - PubMed
-
- Kato, M. & Uchida, J. Recent advances in immune checkpoint inhibitors in the treatment of urothelial carcinoma: A review. Int J. Urol Dec.30 (12), 1068–1077. 10.1111/iju.15278 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

